Abstract
Objective:
To estimate the prevalence of anaemia in Brazilian children up to 83·9 months old.
Design:
Systematic review and meta-analysis, using databases PubMed, Scopus, SciELO, Lilacs, Google Scholar, Periódicos Capes, Arca, Biblioteca Virtual em Saúde, Microsoft Academic Search and Cochrane Library using search terms: anaemia, prevalence, child and Brazil. PROSPERO Registration number: CRD42020208818.
Setting:
Cross-sectional, cohort, case–control and intervention studies published between 2007 and 2020 were searched, excluding those who assessed children with an illness or chronic condition. The main outcome was anaemia prevalence. Random effects models based on the inverse variance method were used to estimate pooled prevalence measures. Sensitivity analyses removed studies with high contribution to overall heterogeneity.
Participants:
From 6790 first screened, 134 eligible studies were included, totalling 46 978 children aged zero to 83·9 months analysed, with adequate regions representativeness.
Results:
Pooled prevalence of anaemia was 33 % (95 % CI 30, 35). Sensitivity analyses showed that withdrawal of studies that contributed to high heterogeneity did not influence national average prevalence.
Conclusions:
Childhood anaemia is still a serious public health problem in Brazil, exposing 33 % of Brazilian children to the anaemia repercussions. The main limitation of the study is the estimation of national prevalence based on local surveys, but a large number of studies were included, with representation in all regions of the country, giving strength to the results. In Brazil, more public policies are needed to promote supplementation, fortification and access to healthy eating to reduce the high level of anaemia among children.
Keywords: Anaemia, Infant, Child, Preschool, Prevalence, Iron deficiency, Brazil
Anaemia is a disease that affects the production of erythrocytes and has as main characteristic the insufficient oxygenation capacity of tissues due to the lower amount of circulating Hb. This phenomenon can occur as a consequence of decreased production and/or increased loss of erythrocytes, with underlying and often overlapping causes(1). This is a global public health problem that affects populations of different socio-economic levels and in all age groups, being more prevalent in poverty regions(2), where there is interaction of factors such as: (a) insufficient intake of Fe of adequate bioavailability (especially that from meat and offal); (b) diets rich in cereals composed of phytates, polyphenols and other ligands that impair intestinal absorption of Fe and (c) poor hygienic and sanitary conditions with parasitic diseases and frequent inflammatory processes(3).
The WHO considers anaemia as an indicator of nutritional and health poverty, which compromises quality of life and contributes to infant mortality. At population level, an anaemia prevalence >4·9 % is considered of public health significance, with a prevalence >40 % classified as a severe public health problem(4). It is estimated that worldwide, the prevalence of anaemia among children in the preschool age group is 47·4 %, ranging from 21·7 % in Europe to 67·6 % in some countries of the African continent(5).
Chronic childhood anaemia is responsible for a number of well-documented physical, emotional and cognitive impairments, such as growth slowdown; pubertal retardation; impaired visual, auditory and memory functions; negative effects on cognitive development and behavioural disorders (appetite perversion, attention deficit hyperactivity, restless leg syndrome)(1,6–9). In addition, the chronic effects of Fe deficiency can also compromise immunity, increasing the risk of infectious diseases and their complications(10).
Brazil nationwide data are not available due to the absence of research studies involving population Hb dosage. Some authors, such as Jordão et al. (11), Iglesias Vásquez et al. (12) and Ferreira et al. (13), have used statistical strategies to estimate national prevalence based on local studies. Currently, surveys of anaemia prevalence conducted in specific localities, of local scope, are rarely published in journals indexed in the most relevant databases and are usually published in smaller journals, although official and with peer review process. Therefore, in order to comprehensively estimate the national prevalence, it is necessary to include in the review the local data published in these journals of lesser expression.
The present study proposes to estimate the Brazilian prevalence of anaemia among infants and preschoolers through a systematic review study with meta-analysis, with the inclusion of data obtained in articles published in official scientific journals, which have International Standard Serial Number and meet the criteria described in the methodology.
Methods
Study design and search strategy
This meta-analysis was undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(14). Studies on the prevalence of anaemia were researched in Brazilian children from birth to 83·9 months, published between January 2007 and July 2020, in the databases PubMed, Scopus, SciELO, Lilacs, Google Scholar, Periódicos Capes, Arca, Biblioteca Virtual em Saúde, Microsoft Academic Search and Cochrane Library.
The choice was made to evaluate the studies published after 2007 in order to be able to verify and update the prevalence estimated in a meta-analysis by Jordão et al. (11), who reviewed studies published between 1996 and 2007. We chose the age group to achieve greater comparability with other studies, since most surveys related to the prevalence of anaemia in Brazil have included children under the age of 7 years.
The terms that used for searching were: ‘anaemia’ ‘iron deficiency anaemia’ and ‘prevalence’ and ‘child’ and ‘Brazil’. These terms in Portuguese were also used: ‘anaemia’, ‘anaemia ferropriva’ and ‘prevalência’ and ‘criança’ and ‘Brasil’. Additional eligible studies on the prevalence of anaemia in childhood were sought by reviewing the reference lists of identified articles and searching relevant journals related to our research topic. No language restrictions were adopted during the search.
The choice to carry out a meta-analysis was made due to the fact that there are a large number of local surveys of anaemia prevalence in Brazil, but that these could only be used to estimate national prevalence through appropriate statistical methods. Although meta-analyses are often criticised for combining heterogeneous data, in the present study, this would have little chance of occurring because data on the prevalence of anaemia were obtained through standardised methods and cut-off points, enabling studies to be analysed together. Additionally, as described (at page 98) by Grant and Booth(15), “small or inconclusive studies lacking in statistical significance can nevertheless make a contribution to the larger picture and such compilations are time efficient for decision makers, particularly when compared with the time taken to review scattered individual studies”.
Study selection and eligibility criteria
Two reviewers independently screened titles and abstracts and critically reviewed the full texts of all selected studies on the basis of the inclusion and exclusion criteria. Any disagreement that arose between reviewers was resolved through discussion and involvement of a third reviewer.
Observational studies (cohort, case–control and cross-sectional studies) and clinical trials were included. In cohort, case–control and intervention studies, the prevalence of anaemia was obtained only at the beginning of the study (baseline). The data were considered only for children residing in Brazil. Inclusion criteria were studies that contained information about the children’s age, city and region of the country, sample size, criteria for defining anaemia and specific method of laboratory evaluation of blood Hb. The cut-off point for defining anaemia should be in accordance with the definitions of the WHO(16). Although the authors’ main interest was in Fe deficiency anaemia, all studies evaluating the prevalence of anaemia were included, even those in which there was no proof that the condition was effectively due to Fe deficiency. It is important to state that, in Brazil, the vast majority of cases of anaemia occur due to Fe deficiency(17). Review studies, studies with secondary data, theses, dissertations, course completion papers, annals of scientific events and studies that evaluated the prevalence of anaemia in children with an illness or chronic condition, hospitalised or not were excluded.
Data extraction and risk of bias/quality assessment
Two authors independently extracted data on the year of the study and the region of the country, the study design, sample size, children’s age group, prevalence and diagnostic criteria for anaemia. The Modified Newcastle–Ottawa quality assessment scale for observational studies (cohort, case–control and cross-sectional studies)(18,19) was used to assess the quality of the study for inclusion. The total score for the Modified Newcastle–Ottawa scale for observational studies is nine stars as a maximum for the overall scale with the minimum of zero. A study was considered of high quality if it reached 7 to 9, medium if it reached 4 to 6 and low if it reached 0 to 3.
For the evaluation of risk-of-bias (RoB) for the non-randomised clinical trials, the ROBINS-I tool (Risk of Bias in Non-randomized Studies-of Interventions) was used(20). The evaluated criteria were divided into pre-intervention, intervention and post-intervention categories. The overall RoB judgement was individually analysed for each study and classified as low, moderate, serious, critical or no information. For randomised clinical trials, the RoB 2 tool (Revised Cochrane risk-of-bias tool for randomised trials)(21) was used, analysing five domains. The overall RoB judgement for each study was classified as low risk, some concerns or high risk.
All studies were included regardless of study quality, in order to obtain the largest possible national coverage of the prevalence of anaemia in childhood. In addition, although some tools are focused on the quality of the intervention, our objective was only to know the prevalence of anaemia in the baseline data. In this case, the representativeness of the sample was more important than the general quality of the intervention. Sensitivity analyses were undertaken to withdraw studies that contributed to high heterogeneity.
Data analysis and heterogeneity assessment
Meta-analysis on the prevalence of anaemia was carried out by using the ‘meta’ package(22) implemented in the R software version 3.6.2. Forest plots including 95 % CI calculated by the Clopper–Pearson exact method were used to describe the prevalence estimates for each study included in the meta-analysis(23). Cochran’s Q test, the between-study variance (tau 2) and I 2 statistics were used to assess heterogeneity(24). Higher values of I 2 indicate a greater degree of heterogeneity among studies. Random effects models based on the inverse variance method were used to estimate pooled prevalence measures, taking into account the high heterogeneity observed between studies. Stratified analyses were performed by Brazilian regions, in order to assess geographic variations of the prevalence of anaemia. The contribution of each study to the overall prevalence measure and heterogeneity was assessed graphically by constructing a Baujat plot(25). Sensitivity analyses removed studies with high contribution to overall heterogeneity, detected by the Baujat plots, in order to assess the possible effects of these studies on the pooled prevalence measures. The sensitivity analyses also assessed the possible effect of studies with sample size <100 and studies that are not cross-sectional on the pooled estimates(26). An alternative funnel plot was used to explore the possibility of publication bias(27). Alternative funnel plots are constructed using study size rather than 1/se in y-axis, as recommended by Hunter et al. (27) in meta-analyses of proportion studies. Funnel plots are skewed and asymmetrical in the presence of publication bias and other biases.
The data presented are stratified by regions of Brazil and consolidated with the estimate of national prevalence. Brazil is divided into five geographic regions, being: North, Northeast, Midwest, Southeast and South. Data for the North and Midwest regions were grouped. This option was adopted due to some aspects described below: (1) fewer studies identified in these regions; (2) similar geographical and economic characteristics, such as the smaller number of large urban agglomerates, the presence of extensive areas of vegetation and watersheds, a smaller and less populous coastal strip, the presence of native indigenous populations and low migration and (3) and no studies were found regarding the metropolitan region of the Federal District, Brasília (located in the Midwest region), which could contribute to data asymmetry due to cosmopolitan characteristics similar to those observed in the metropolises of the South and Southeast regions.
Study registration
The protocol for this review was registered with PROSPERO: (no. CRD42020208818)
Results
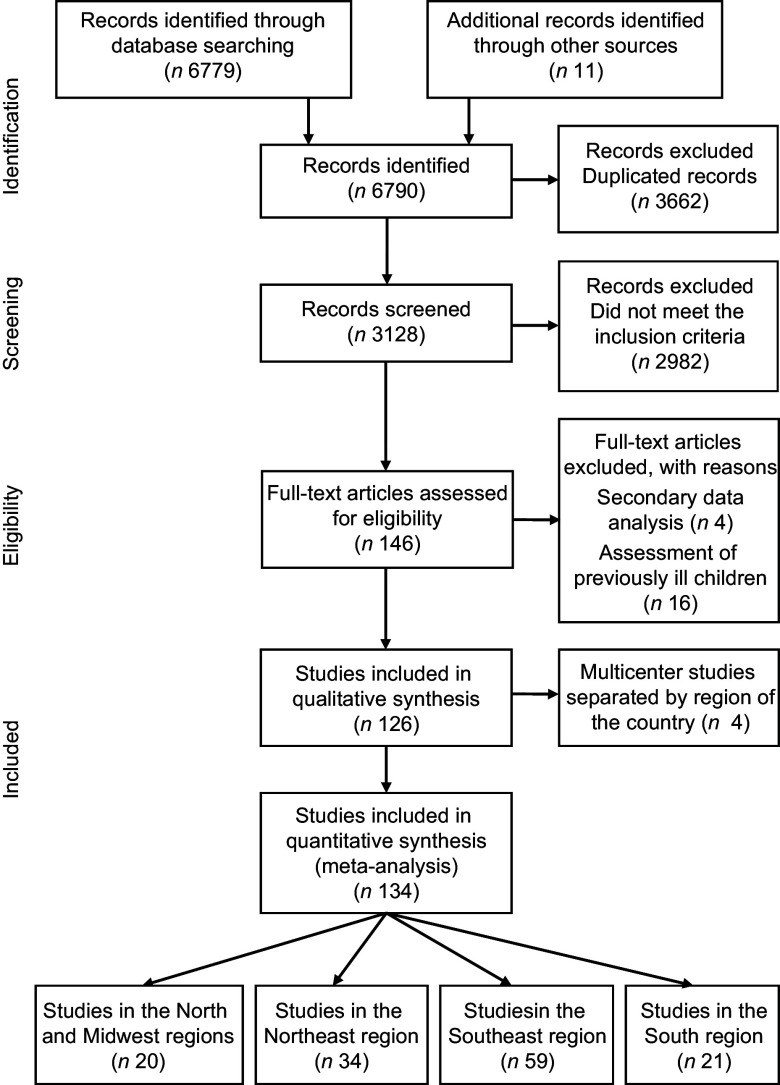
The search of articles in the databases identified 6779 records from January 2007 to July 2020. An additional eleven articles were identified from reference lists and hand searches. Of these, 3128 records remained after the removal of duplicates (Fig. 1). Based on the title and screening of the abstract, 2982 records were removed due to not meeting the inclusion criteria. In total, 146 full-text articles were reviewed. In this full-text screening, twenty articles were excluded due to the analyses of secondary data and evaluation of previously ill children (Fig. 1). A total of 126 papers met the inclusion criteria and were included in the meta-analysis. As some of these articles were multicentre studies, the prevalence of anaemia was presented separately for each region where these studies were conducted. Therefore, the meta-analysis was conducted based on 126 papers and 134 studies, twenty from the North and Midwest regions(28–47), thirty-four from the Northeast region(29,38,39,48–76), fifty-nine from the Southeast region(55,77–134) and twenty-one from the Southern region(66,88,135–153).
Fig. 1.
Flow chart of the selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement
One hundred and seven cross-sectional studies, eight cohort studies, one case–control study and eighteen intervention studies were included (see online Supplemental Table 1). The number of subjects per study ranged from 31 to 2376, with a mean age that ranged from 0 to 83·9 months. The Newcastle–Ottawa scale for observational studies was applied to 116 studies. Thirty-nine studies were classified as high quality, seventy-six as medium quality and one was classified as low quality of evidence. The ROBINS-I tool was used in nine studies. Five studies showed a moderate risk and four studies a serious RoB. The RoB 2 tool was used in nine studies, of which four were assessed as low RoB, four with some concerns and one with high risk (see online Supplemental Table 1).
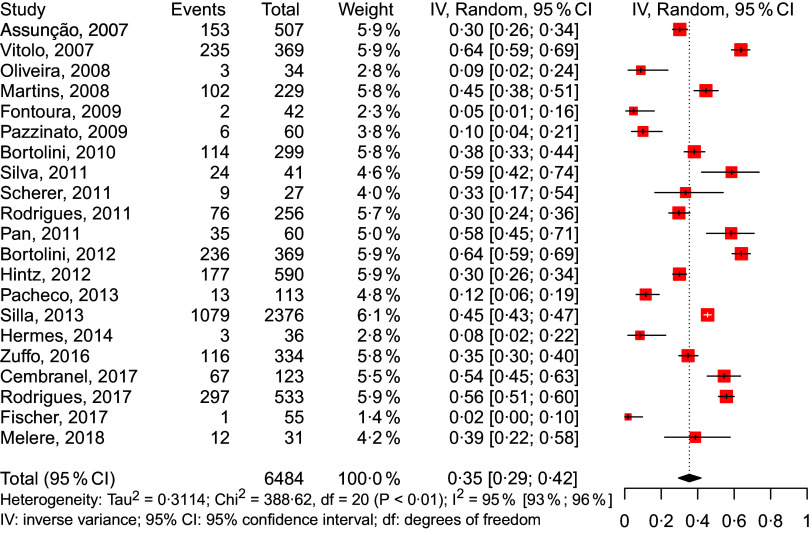
In total, 46 978 children were included in the study, with an estimated national prevalence of 33 % of anaemia (Fig. 2). The prevalence measured was similar for the North/Midwest (36 %), Northeast (38 %) and South (35 %); however, they were lower in the Southeast region (28 %). Figures 3–6 show the results obtained according to the different regions.
Fig. 2.
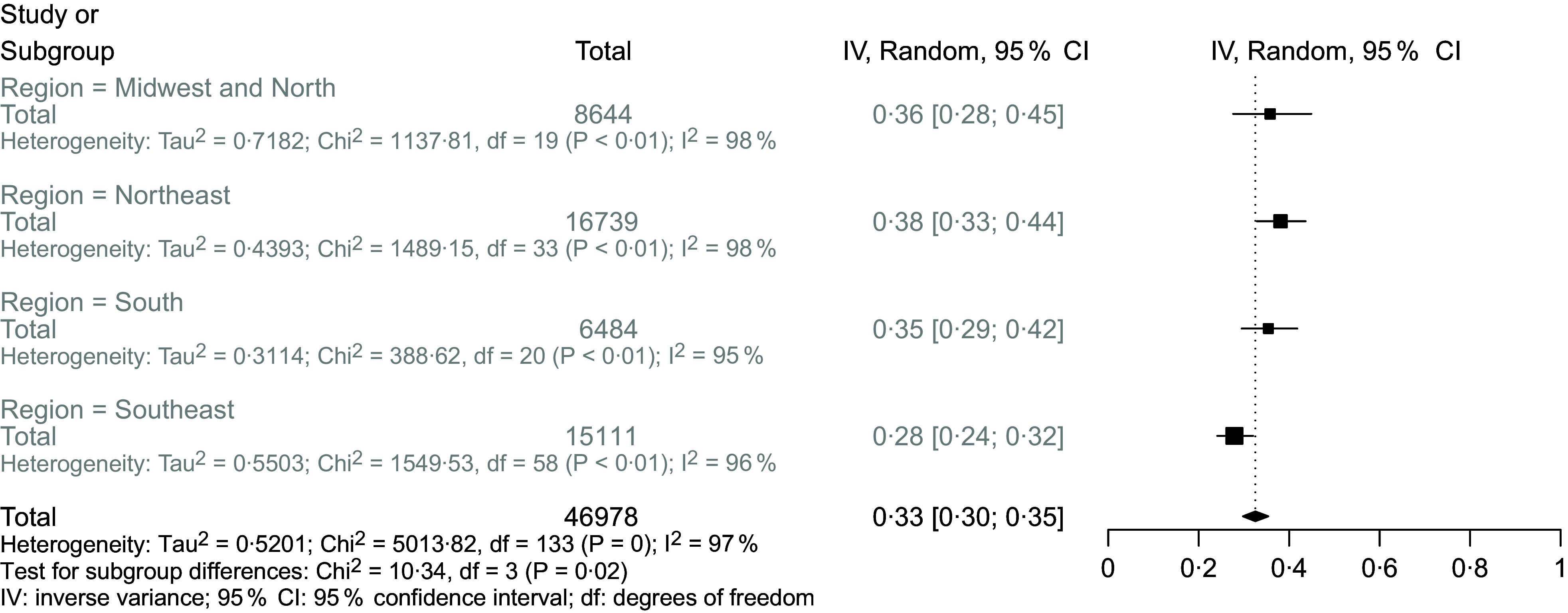
Forest plot for meta-analysis of the prevalence of anaemia in all Brazilian regions
Fig. 3.
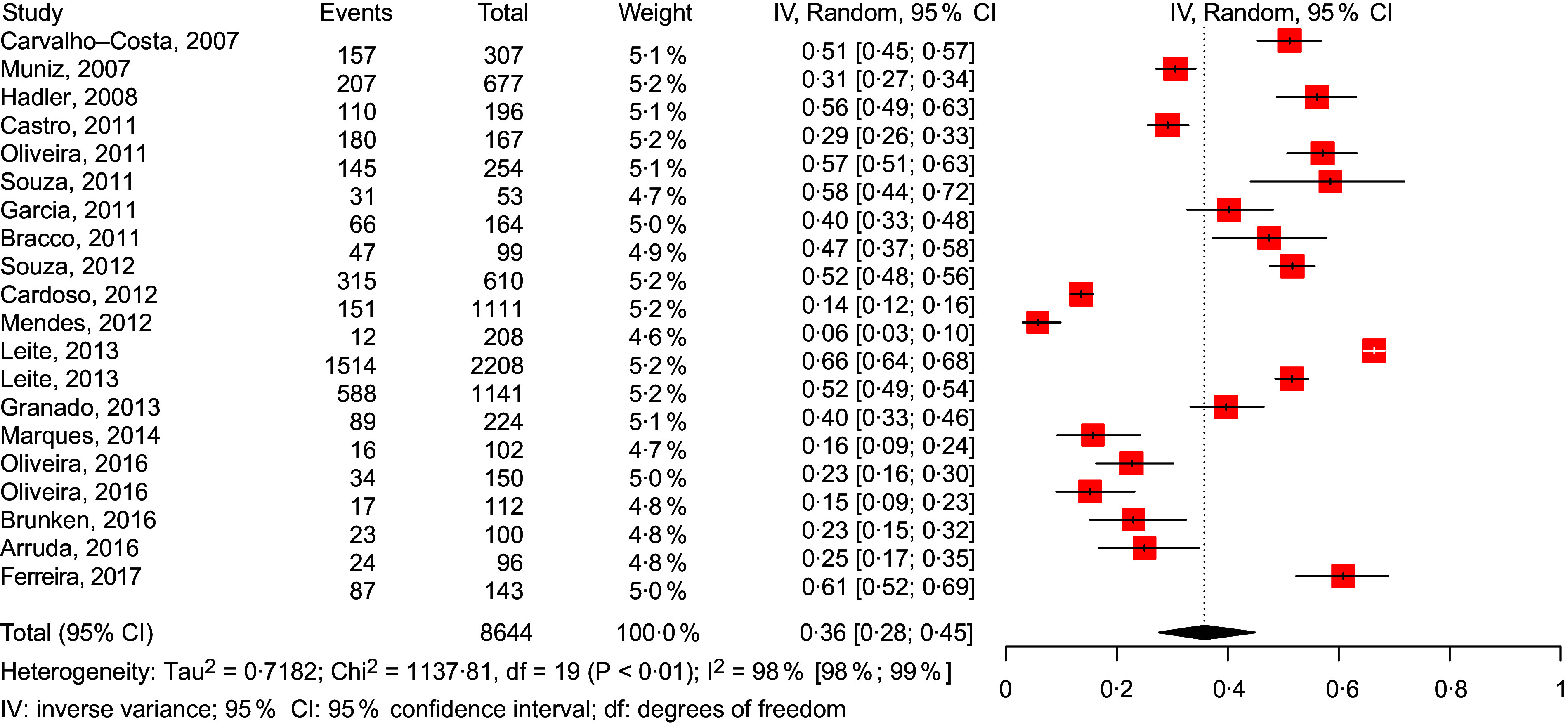
Forest plot for meta-analysis of the prevalence of anaemia in the Brazilian Midwest and North regions (ordered by year of publication)
Fig. 6.
Forest plot for meta-analysis of the prevalence of anaemia in the Brazilian South region (ordered by year of publication)
One of the aspects that can be observed by the disposition of the studies in the graphs, which are presented according to the date of publication, is the fact that there seems to be no temporal trend, between 2007 and 2020, of changes in prevalence.
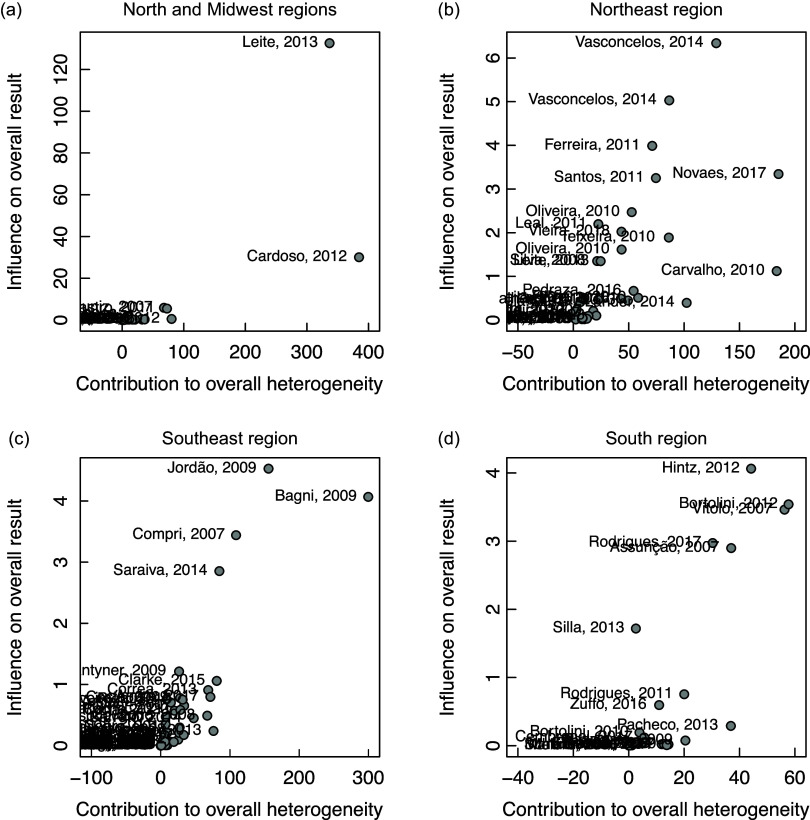
Figure 7 shows the contribution of each study to the overall effect size by Baujat plots, where the impact of excluding a study from the final analyses (vertical axis) is plotted against its contribution to the heterogeneity statistic (horizontal axis). For example, panel (a) of Fig. 7 shows that the studies by Leite et al. (2013)(39) and Cardoso et al. (2012)(31) have higher contribution to the overall heterogeneity and high influence to the overall result and, consequently, it is important to evaluate the effect of the removal of these studies in sensitivity analyses. The sensitivity analyses in Table 1 show that the removal of these studies did not significantly influence the average prevalence of each region, except in the South region.
Fig. 7.
Contribution of each study to the overall effect size by Baujat plots
Table 1.
Sensitivity analyses table
| Studies | Prevalence | 95 % CI | I 2 (%) | Cochran’s Q | |
|---|---|---|---|---|---|
| North and Midwest regions | |||||
| Removal of studies with sample size <100 | 17 | 34·6 | 25·9, 44·6 | 98·6 | 1118·01 |
| Removal of two studies with high contribution to overall heterogeneity | 18 | 36·1 | 29·8, 43·1 | 95·6 | 385·14 |
| Removal of studies that are not cross-sectional | 17 | 35·5 | 26·6, 45·5 | 98·5 | 1094·48 |
| All studies | 20 | 35·8 | 27·6, 44·9 | 98·3 | 1137·81 |
| Northeast region | |||||
| Removal of studies with sample size <100 | 27 | 37·1 | 31·4, 43·1 | 98·2 | 1407·95 |
| Removal of two studies with high contribution to overall heterogeneity | 32 | 37·3 | 32·6, 42·3 | 97·2 | 1119·81 |
| Removal of studies that are not cross-sectional | 27 | 37·6 | 31·7, 43·8 | 98·1 | 1337·50 |
| All studies | 34 | 38·1 | 32·8, 43·6 | 97·8 | 1489·15 |
| Southeast region | |||||
| Removal of studies with sample size <100 | 45 | 29·8 | 25·3, 36·6 | 97·0 | 1647·47 |
| Removal of two studies with high contribution to overall heterogeneity | 57 | 26·4 | 23·1, 29·9 | 94·8 | 1076·43 |
| Removal of studies that are not cross-sectional | 49 | 27·0 | 23·3, 31·0 | 95·8 | 1133·22 |
| All studies | 59 | 27·9 | 24·1, 32·0 | 96·3 | 1549·53 |
| South region | |||||
| Removal of studies with sample size <100 | 12 | 41·3 | 34·2, 48·7 | 96·4 | 305·14 |
| Removal of six studies with high contribution to overall heterogeneity | 15 | 33·8 | 27·7, 40·4 | 88·7 | 124·11 |
| Removal of studies that are not cross-sectional | 17 | 30·4 | 24·7, 56·8 | 92·3 | 209·04 |
| All studies | 21 | 35·4 | 29·5, 41·8 | 94·9 | 388·62 |
| Brazil | |||||
| Removal of studies with sample size <100 | 101 | 33·8 | 30·7, 37·1 | 97·9 | 4713·40 |
| Removal of all studies with high contribution to overall heterogeneity in each region | 122 | 31·9 | 29·4, 34·4 | 96·1 | 3105·42 |
| Removal of studies that are not cross-sectional | 110 | 31·1 | 28·2, 34·1 | 97·4 | 4170·68 |
| All studies | 134 | 32·5 | 29·8, 35·4 | 97·3 | 5013·82 |
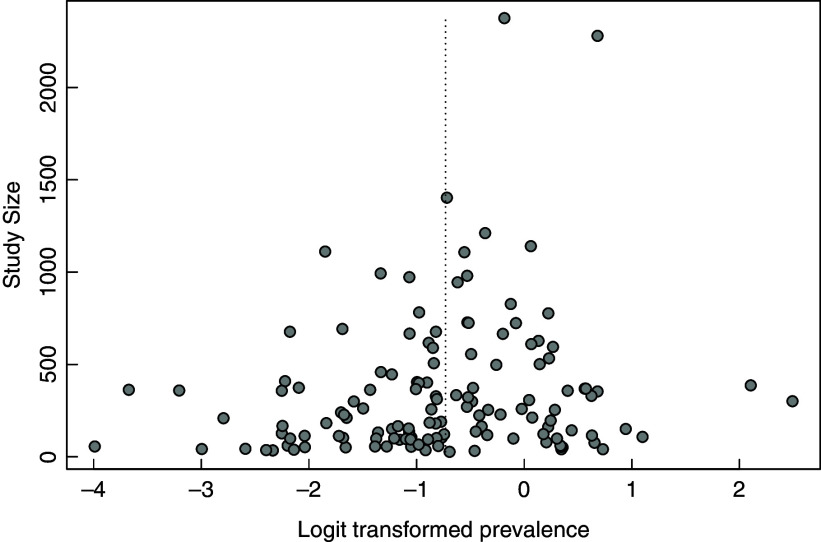
The funnel plot for publication bias analyses considering the 134 studies is showed in Fig. 8. This graph seems to suggest that there is a reasonably symmetrical distribution of the logit transformed prevalence estimates around the pooled prevalence, indicating that this systematic review is not subject to a publication bias(26).
Fig. 8.
Funnel plot for publication bias analyses considering the 134 studies. The dashed vertical line refers to the position of the pooled prevalence in Brazil
Discussion
Brazil is a country of continental dimensions and has faced successive political and economic crises. These facts, in a way, have interfered in the production of information and data related to health conditions of national scope. In the nutritional area, even relatively simple information, such as nutritional status obtained by anthropometry, is scarce in childhood and the last national survey that showed prevalence of malnutrition was conducted in 2008(154). Regarding anaemia, there are virtually no national data that have been obtained by a study designed for this purpose, including representative sampling from all over the country. An attempt to estimate was made in 2006, in Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher (PNDS 2006)(155), in which 3499 blood samples from children under 5 years of age using the dry drop technique were analysed, and Hb values below 11 g/dl were considered as anaemia. However, a number of methodological limitations, including loss of samples and the fact that the dry drop method was not validated for children, led to the results of this study being questioned(156–158). Additionally, these data are currently out dated in about 14 years(155).
For this reason, given the need to know the information about the prevalence of anaemia in the country as a whole and in its regions, in order to structure control measures, some authors have sought to estimate this information based on local studies(11–13). One of the first initiatives in this sense appeared in the literature in 2009, by Jordão et al. (11), who reviewed fifty-three studies published between 1996 and 2007 and, through meta-analysis, estimated a national prevalence of 53 % of anaemia among children aged between zero and 5 years. Due to the large number of affected children, numerous attempts at fortification were tested in the country, especially studies by the Dutra-de-Oliveira group that sought to fortify drinking water with Fe, considering that to reach all children exposed to anaemia, it would be necessary to fortify food of widespread consumption(114,159,160). An aspect that may be relevant for the prevalence has fallen, considering the data from Jordão et al. (11), and those in the present study refer to the possible impact of government initiatives to combat Fe deficiency. Because of a law published in 2002, from 2004, fortification of wheat and maize flours with Fe and folic acid became mandatory in Brazil(161) and, in 2005, the Saúde de Ferro programme was implemented, which added initiatives for universal supplementation of children up to 2 years old(162). These two initiatives may have contributed to the decrease in prevalence observed in these two studies of similar methodology, but covering sequential periods. On the other hand, our meta-analysis showed that, at least between 2007 and 2020, there seems to have been no trend of change in prevalence. The results are found, as can be seen in the graphs referring to the regions, scattered over the years, not seeing a propensity to fall or increase in numbers.
More recently, Iglesias Vásquez et al. (12) found a prevalence of 39·6 % of anaemia among preschool children and schoolchildren in Latin America, reviewing eighteen studies published between 2000 and 2014. And, Ferreira et al. (13) reviewed thirty-seven studies published between 2007 and 2019 involving 17 741 Brazilian children aged 6–60 months and found, through meta-analysis, a general prevalence of 40·4 % of anaemia. The article by Ferreira et al. (13) aimed to describe the prevalence of anaemia in different scenarios (health services, populations subject to social inequalities and population-based studies), including thirty-seven studies with children aged 6–60 months. Our analysis included 134 studies, with children from a wider age group and also differed by describing the prevalence according to the regions of the country. These regional inequalities have not been studied in previous meta-analyses(13).
The present study, when compared with the three mentioned above(11–13), who evaluated similar age groups, found a lowest prevalence. However, considering that the number of studies included was quite high, it is possible that our data are better able to reflect the real situation. In any case, the figures are extremely high in view of the recognised repercussions of anaemia(163–165) and the situation demonstrated has the potential to compromise the growth and development of about 1/3 of the country’s children.
Fe deficiency has been shown to compromise thyroid function and negatively influence growth(166–169). According to Pivina et al., Fe deficiency can cause changes in neurotransmitter homoeostasis, decrease myelin production, impair synaptogenesis and decrease basal ganglia function, negatively affecting cognitive functions and psychomotor development, and is also frequent comorbidity in autism and attention deficit hyperactivity disorder(170). Several studies have proven that Fe deficiency anaemia in childhood due to its negative effects mainly on myelogenesis and synaptogenesis(9) is associated with changes in development and motor skills and worse cognitive performance in varying degrees (results identified through assessments performed with the Denver II scale), which certainly compromises the learning and, consequently, the permanent intellectual future of these children(163,170–173). Fe deficiency, interfering in the production and action of cytokines, reducing the phagocytic capacity of neutrophils and macrophages(174) and compromising the production of T lymphocytes, particularly CD4 + Th1 subpopulations(175), has been associated with changes that compromise the proper functioning of both the innate and adaptive immune systems(176,177), increasing susceptibility to infections by intracellular microorganisms. Among the various roles played by Fe in the body should be mentioned the modulating function of the innate immune response(178) which is impaired when the levels of this metal are decreased, predisposing the body to infection(179,180).
Historically, Brazil has been unable to effectively reduce its prevalence of Fe deficiency anaemia. Some factors can be listed as possibly responsible for this fact, and one of them dates back to the first year of life, when the use of unmodified cows’ milk is used by 62·4 % of babies aged 0–5 months and 74·6 % of those between 6 and 12 months, when the child is weaned(181). Due to the fact that it has little amount of low bioavailability Fe and the chelating potential of milk Ca; and leading to micro haemorrhages in the intestinal mucosa, cows’ milk contributes decisively to the installation and maintenance of Fe deficiency(182,183). Many babies are not able to satisfactorily make up their reserves during the gestational period and even during breast-feeding because the prevalence of anaemia among pregnant and lactating women is also high in the country(184). It should also be remembered the low use of fortified foods during the feeding introduction period in Brazilian children(185).
Another aspect concerns the consumption of Fe of inadequate bioavailability, associated with low-cost foods and low nutritional density, characteristic of the poorest countries, in which good sources of Fe, especially meat and fortified foods, have a higher cost(133). Additionally, the diet based on plant protein sources predisposes to the intake of Fe-chelators nutrients, such as phytate, and is usually poor in absorption stimulating nutrients such as vitamin C(186,187). A recent survey published in 2020 conducted by the Brazilian government showed that food insecurity in the country increased by 33·3 % compared with 2004 and 62·2 % compared with 2013, leading to a significant portion of the population being exposed to inadequate and insufficient micronutrient nutrition(188). The same research showed that in the North and Northeast regions, less than half of the households in these regions had full and regular access to food and that the general sewage network was present only in about half of the households with moderate and severe food insecurity and, in both cases, the existence of a fossa not connected to the health network was quite relevant (43 %)(188); data from the present study found the highest prevalence of anaemia in these two regions. The issue of basic sanitation of very low coverage in the country can also contribute to the difficulty of controlling anaemia, as it provides a greater amount of infectious diseases and intestinal parasitosis(189). At the other end of this issue, it is verified that the prevalence of anaemia has shown a slight fall in southeast Brazil in the last 10 years (Fig. 5), which can be explained by the better socio-economic conditions of this region in relation to the others. The Southeast region has the highest Human Development Index(190) and the highest rates of access to basic sanitation in the country(191); in addition, with the exception of the South region, the Southeast has the lowest rates of food insecurity in Brazil(188).
Fig. 5.
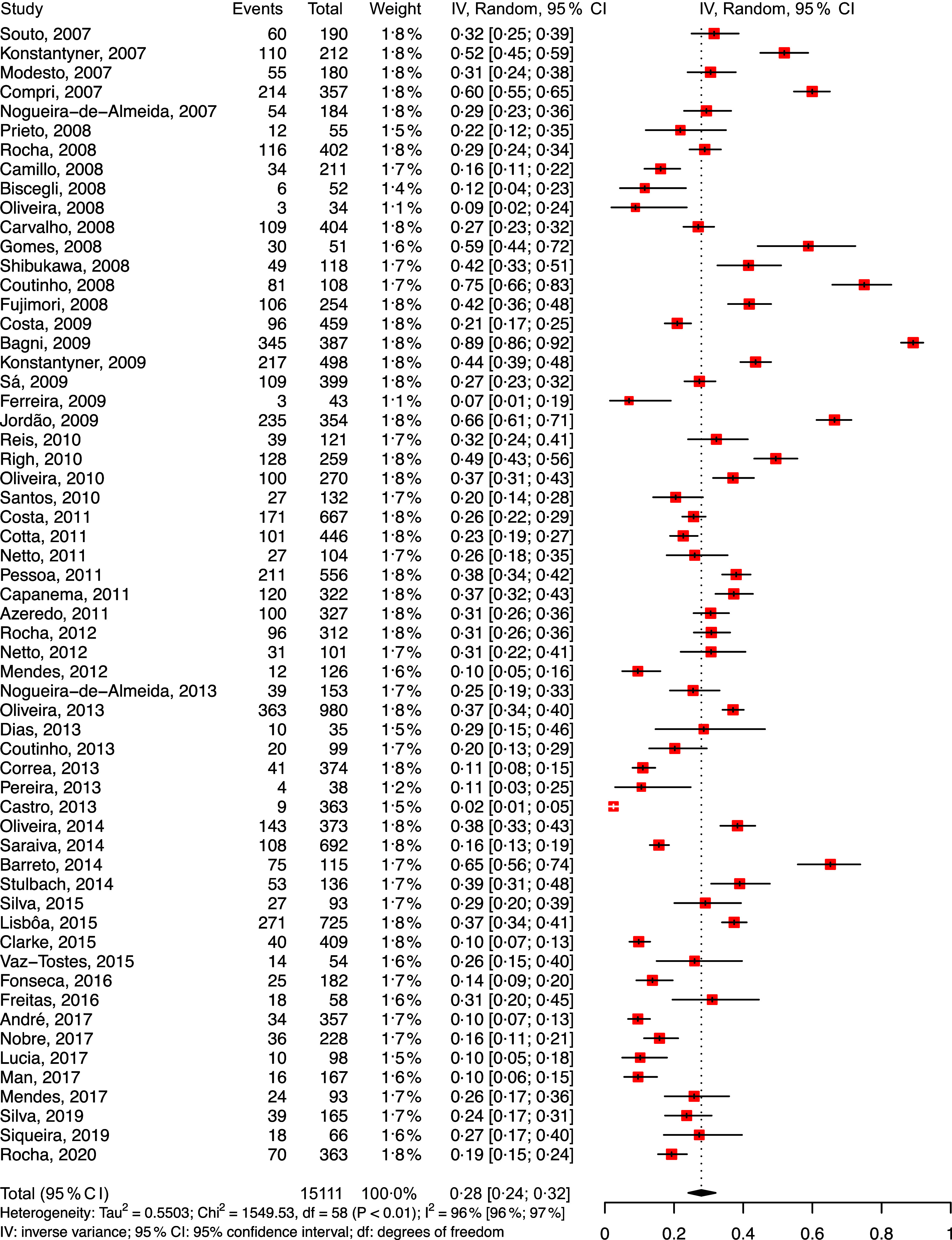
Forest plot for meta-analysis of the prevalence of anaemia in the Brazilian Southeast region (ordered by year of publication)
Some aspects may have interfered in the results of the present study. There is a tendency for research on a given disease to focus on areas where it is most prevalent. Thus, it is likely that some results, especially those with abnormally high prevalence of anaemia, have been obtained exactly in regions where high results would already be expected. Similarly, areas with more socio-economically privileged populations may have been less investigated than the poorer ones. The data in Figs. 2–6 show that an asymmetric arrangement of the points was observed, demonstrating that this fact must have actually occurred(192). For this to be avoided, it would be necessary to study with national sampling, which is not available in Brazil, justifying the search for results that are obtained in another way.
Fig. 4.
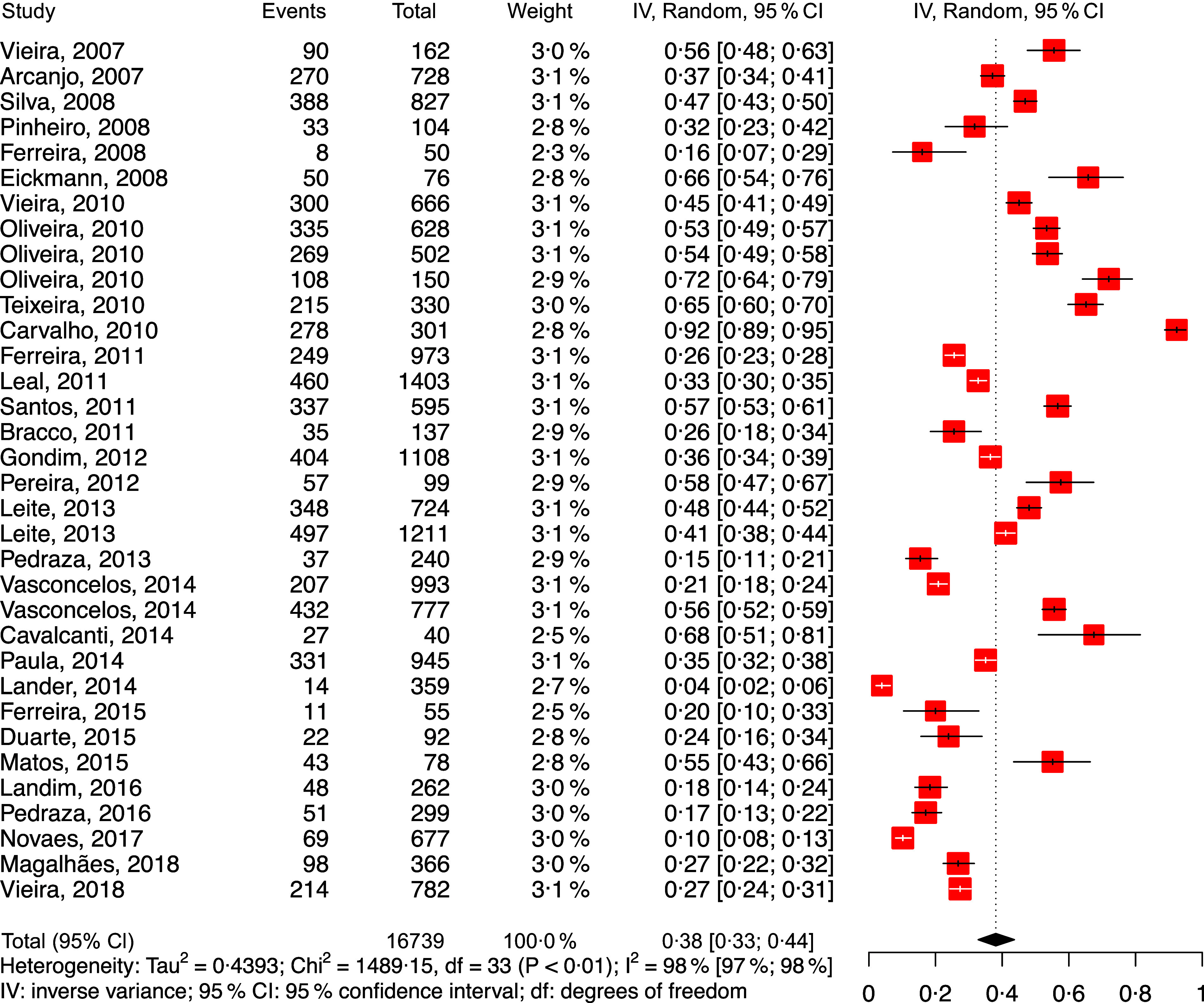
Forest plot for meta-analysis of the prevalence of anaemia in the Brazilian Northeast region (ordered by year of publication)
Joint analyses of cross-sectional and longitudinal studies may include bias in the results. However, as shown in Table 1, the impact of the separation of these studies was not relevant for the final results. Similarly, the sample size was also quite different in each of the studies. However, Table 1 shows that the exclusion of studies with low sample size did not alter the prevalence of anaemia in the regions, except in the South region, in which, with drawing studies with sample size smaller than 100, the prevalence increases from 35·4 to 41·3 %. The careful analyses of the individual studies considered showed the existence of outliers, which can be verified in Fig. 7. As can be seen in Table 1, the withdrawal of these surveys practically does not alter the results.
The present study has some limitations. The first, and more importantly, refers to the fact that, considering that local prevalence studies are rarely published in the most important scientific journals, in so that the survey could be made feasible, it was necessary to also seek data in journals of lesser expression. This method may have led to the inclusion of data with a lower degree of reliability, even taking care to include only articles in which quality was verified. Another limitation concerns the fact that almost all studies that seek to determine the prevalence of Fe deficiency anaemia do not include criteria that prove that there is nutritional deficiency. Therefore, the results presented here may include anaemia for causes other than deficiency of Fe food intake. However, knowing that in low- and middle-income countries, the vast majority of cases have nutritional origin and, taking into account that studies with children with an illness condition were not included, this limitation can be considered of little relevant impact. Five studies did not report the Hb cut-off point used to define anaemia, and it was not possible to obtain this information by direct contact with the authors. However, the methodology reported that internationally accepted criteria were used, so that the cut-off points suggested by the WHO were considered to have been used. Finally, it should also be noted that not all studies used the same method of laboratory evaluation, including surveys with different analyses techniques, even with portable methodologies.
Conclusion
Our meta-analysis of 134 studies revealed a high prevalence of anaemia among Brazilian preschool children. Although the comparison with previous similar studies suggests a reduction in prevalence, the numbers are still very high, placing approximately one-third of Brazilian children at risk of damage to physical and psychosocial health. There is an urgent need for the Brazilian government to conduct a nationwide survey of Fe deficiency, using appropriate biomarkers, in order to confirm whether the prevalence is really so high and, if so, implement appropriate public health strategies.
Acknowledgements
Acknowledgements: None. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: There are no conflicts of interest. Authorship: C.A.N.-d.-A.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; figures, study design; data collection; data interpretation; data analyses. F.d.V.U.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; figures, study design; data collection; data interpretation. L.A.D.C.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; data interpretation; data analyses. E.Z.M.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; data interpretation; data analysis; figures; meta-analyses. I.S.F.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; data interpretation. A.A.C.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; literature search; data analysis; data interpretation. F.C.S.d.C.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; data collection; data analysis. R.F.B.S.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; literature search; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; data collection. M.E.N.-d.-A.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; literature search. J.A.L.: Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; literature search; data analysis; data interpretation. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100286X.
click here to view supplementary material
References
- 1. Allali S, Brousse V, Sacri AS et al. (2017) Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol 10, 1023–1028. [DOI] [PubMed] [Google Scholar]
- 2. WHO (2017) Nutritional Anaemias: Tools for Effective Prevention and Control No. 9241513063. Geneva: World Health Organization. [Google Scholar]
- 3. Hunt JR (2003) Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 78, 633S–639S. [DOI] [PubMed] [Google Scholar]
- 4. WHO (2008) Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. WHO Global Database on Anaemia No. 9241596651. Geneva: World Health Organization. [Google Scholar]
- 5. De Benoist B, Cogswell M, Egli I et al. (2008) Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database of Anaemia. Geneva: World Health Organization. [Google Scholar]
- 6. Lozoff B, Clark KM, Jing Y et al. (2008) Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr 152, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lukowski AF, Koss M, Burden MJ et al. (2010) Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 13, 54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattiello V, Schmugge M, Hengartner H et al. (2020) Diagnosis and management of iron deficiency in children with or without anemia: consensus recommendations of the SPOG Pediatric Hematology Working Group. Eur J Pediatr 179, 527–545. [DOI] [PubMed] [Google Scholar]
- 9. Vallée L (2017) Iron and neurodevelopment. Arch Pediatr 24, 5s18–5s22. [DOI] [PubMed] [Google Scholar]
- 10. Tansarli GS, Karageorgopoulos DE, Kapaskelis A et al. (2013) Iron deficiency and susceptibility to infections: evaluation of the clinical evidence. Eur J Clin Microbiol Infect Dis 32, 1253–1258. [DOI] [PubMed] [Google Scholar]
- 11. Jordão RE, Bernadi JL & Barros Filho AA (2009) Prevalence of iron-deficiency anemia in Brazil: a systematic review. Rev Paul Pediatr 27, 90–98. [Google Scholar]
- 12. Iglesias Vazquez L, Valera E, Villalobos M et al. (2019) Prevalence of anemia in children from Latin America and the Caribbean and effectiveness of nutritional interventions: systematic review and meta-analysis. Nutrients 11, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira HS, Vieira RCS, Livramento ARS et al. (2021) Prevalence of anaemia in Brazilian children in different epidemiological scenarios: an updated meta-analysis. Public Health Nutr 24, 2171–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant MJ & Booth A (2009) A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 26, 91–108. [DOI] [PubMed] [Google Scholar]
- 16. WHO (2011) Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization. [Google Scholar]
- 17. Coutinho GGPL, Goloni-Bertollo EM & Bertelli ÉCP (2005) Iron deficiency anemia in children: a challenge for public health and for society. Sao Paulo Med J 123, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells GA, Shea B, O’Connell D et al. (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed September 2020).
- 19. Modesti PA, Reboldi G, Cappuccio FP et al. (2016) Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One 11, e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Hernán MA, Reeves BC et al. (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JAC, Savović J, Page MJ et al. (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. [DOI] [PubMed] [Google Scholar]
- 22. Schwarzer G (2007) Meta: an R package for meta-analysis. R News 7, 40–45. [Google Scholar]
- 23. Andrade C (2020) Understanding the basics of meta-analysis and how to read a forest plot: as simple as it gets. J Clin Psychiatry 81, 20f13698. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baujat B, Mahé C, Pignon J-P et al. (2002) A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med 21, 2641–2652. [DOI] [PubMed] [Google Scholar]
- 26. Egger M, Smith GD, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunter JP, Saratzis A, Sutton AJ et al. (2014) In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 67, 897–903. [DOI] [PubMed] [Google Scholar]
- 28. Arruda EFd, Araujo FMd, Guimarães MGdS et al. (2016) Association between malaria and anemia in an urban area with Plasmodium transmission: Mâncio Lima, Acre State, Brazil. Cad Saude Publica 32, e00115514. [DOI] [PubMed] [Google Scholar]
- 29. Bracco MM, Colugnati FAB, Gomes PAP et al. (2011) Evaluation of the technology employed in the Hb-010 Hemoglobinmeter (Agabê®) and the possibility of its application in the Sistema Único de Saúde. Bol Inst Saúde 13, 46–52. [Google Scholar]
- 30. Brunken GS, França GVAd, Luiz RR et al. (2016) Agreement assessment between hemoglobin and hematocrit to detect anemia prevalence in children less than 5 years old. Cad Saúde Colet 24, 118–123. [Google Scholar]
- 31. Cardoso MA, Scopel KK, Muniz PT et al. (2012) Underlying factors associated with anemia in Amazonian children: a population-based, cross-sectional study. PLoS One 7, e36341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carvalho-Costa FA, Gonçalves AQ, Lassance SL et al. (2007) Giardia Lamblia and other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev Inst Med Trop S Paulo 49, 147–153. [DOI] [PubMed] [Google Scholar]
- 33. Castro TGd, Silva-Nunes M, Conde WL et al. (2011) Anemia e deficiência de ferro em pré-escolares da Amazônia Ocidental Brasilia: prevalência e fatores associados. Cad Saude Publica 27, 131–142. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira AA, Santos RV, Souza JAMd et al. (2017) Anemia and hemoglobin levels among Indigenous Xavante children, Central Brazil. Rev Bras Epidemiol 20, 102–114. [DOI] [PubMed] [Google Scholar]
- 35. Garcia MT, Granado FS & Cardoso MA (2011) Complementary feeding and nutritional status of 6-24-month-old children in Acrelândia, Acre State, Western Brazilian Amazon. Cad Saude Publica 27, 305–316. [DOI] [PubMed] [Google Scholar]
- 36. Granado FS, Augusto RA, Muniz PT et al. (2013) Anaemia and iron deficiency between 2003 and 2007 in Amazonian children under 2 years of age: trends and associated factors. Public Health Nutr 16, 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hadler MCCM, Sigulem DM, Alves MdFC et al. (2008) Treament and prevention of anemia with ferrous sulfate plus folic acid in children attending daycare centers in Goiânia, Goiás State, Brazil: a randomized controlled trial. Cad Saude Publica 24, s259–s271. [DOI] [PubMed] [Google Scholar]
- 38. Leite FMdB, Ferreira HdS, Bezerra MKdA et al. (2013) Food intake and nutritional status of preschool from maroon communities of the state Alagoas, Brazil. Rev Paul Pediatr 31, 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leite MS, Cardoso AM, Coimbra CE Jr et al. (2013) Prevalence of anemia and associated factors among indigenous children in Brazil: results from the First National Survey of Indigenous People’s Health and Nutrition. Nutr J 12, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marques RFSV, Taddei JAAC, Lopez FA et al. (2014) Breastfeeding exclusively and iron deficiency anemia during the first 6 months of age. Rev Assoc Med Bras 60, 18–22. [DOI] [PubMed] [Google Scholar]
- 41. Mendes L, Vieira MG, de Melo EM et al. (2012) Prevalence of anemia in children in municipal centers for child education (CMEIS) in the city of Trindade–Goiás. Vita et Sanitas 6, 103–114. [Google Scholar]
- 42. Muniz PT, Castro TGd, Araújo TSd et al. (2007) Child health and nutrition in the Western Brazilian Amazon: population-based surveys in two counties in Acre State. Cad Saude Publica 23, 1283–1293. [DOI] [PubMed] [Google Scholar]
- 43. Oliveira CSdM, Augusto RA, Muniz PT et al. (2016) Anemia and micronutrient deficiencies in infants attending at Primary health Care in Rio Branco, Acre, Brazil. Cien Saude Colet 21, 517–530. [DOI] [PubMed] [Google Scholar]
- 44. Oliveira CSdM, Cardoso MA, Araújo TSd et al. (2011) Anemia in children 6 to 59 months of age and associated factors in Jordão, Acre State, Brazil. Cad Saude Publica 27, 1008–1020. [DOI] [PubMed] [Google Scholar]
- 45. Oliveira CSM, Sampaio P, Muniz PT et al. (2016) Multiple micronutrients in powder delivered through primary health care reduce iron and vitamin A deficiencies in young Amazonian children. Public Health Nutr 19, 3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Souza AT, Faustino SMM & do Nascimento Rodrigues AS (2011) Determination of iron deficiency anemia in children between 03 to 04 years old associated with enteroparasitosis-Macapá-Amapá. Cienc Equat 1, 58–63. [Google Scholar]
- 47. Souza OF, de Macedo LF, de Menezes Oliveira CS et al. (2012) Prevalence and associated factors to anaemia in children. J Hum Growth Dev 22, 307–313. [Google Scholar]
- 48. Arcanjo FPN, Pinto VPT, Coelho MR et al. (2007) Anemia reduction in preschool children with the addition of low doses of iron to school meals. J Trop Pediatr 54, 243–247. [DOI] [PubMed] [Google Scholar]
- 49. Vieira ACF, Diniz AS, Cabral PC et al. (2007) Nutritional assessment of iron status and anemia in children under 5 years old at public daycare centers. J Pediatr 83, 370–376. [DOI] [PubMed] [Google Scholar]
- 50. Eickmann SH, Brito CMM, Lira PIC et al. (2008) Effectiveness of weekly iron supplementation on hemoglobin concentration, nutritional status and development of infants of public daycare centers in Recife, Pernambuco State, Brazil. Cad Saude Publica 24, s303–s311. [DOI] [PubMed] [Google Scholar]
- 51. Ferreira HdS, Cavalcante SdA, Cabral CR Jr et al. (2008) Effects of the consumption of “multimixture” on nutritional status: a community trial involving children from a slum district on the outskirts of Maceió, State of Alagoas, Brazil. Rev Bras Saude Mater Infant 8, 309–318. [Google Scholar]
- 52. Pinheiro FGMB, Santos SLDX, Cagliari MPP et al. (2008) Evaluation of anemia in children from the city of Campina Grande, Paraíba, Brazil. Rev Bras Hematol Hemoter 30, 457–462. [Google Scholar]
- 53. Silva SCLe, Batista Filho M & Miglioli TC (2008) Prevalence and risk factors of anemia among women and their children in the State of Pernambuco. Rev Bras Epidemiol 11, 266–277. [Google Scholar]
- 54. Carvalho AGC, Lira PICd, Barros MdFA et al. (2010) Diagnosis of iron deficiency anemia in children of Northeast Brazil. Rev Saúde Pública 44, 513–519. [DOI] [PubMed] [Google Scholar]
- 55. Oliveira ASd, Silva RdCR, Fiaccone RL et al. (2010) Effect of length of exclusive breastfeeding and mixed feeding on hemoglobin levels in the first six months of life: a follow-up study. Cad Saude Publica 26, 409–417. [DOI] [PubMed] [Google Scholar]
- 56. Oliveira JS, Lira PICd, Osório MM et al. (2010) Anemia, hypovitaminosis A and food insecurity in children of municipalities with Low Human Development Index in the Brazilian Northeast. Rev Bras Epidemiol 13, 651–664. [DOI] [PubMed] [Google Scholar]
- 57. Teixeira Mde L, Lira PI, Coutinho SB et al. (2010) Influence of breastfeeding type and maternal anemia on hemoglobin concentration in 6-month-old infants. J Pediatr 86, 65–72. [DOI] [PubMed] [Google Scholar]
- 58. Vieira RCdS, Ferreira HdS, Costa ACS et al. (2010) The prevalence of and risk factors for anemia in preschool children in the State of Alagoas, in Brazil. Rev Bras Saude Mater Infant 10, 107–116. [Google Scholar]
- 59. Ferreira HdS, Lamenha MLD, Xavier AFS Jr et al. (2011) Nutrition and health in children from former slave communities (quilombos) in the state of Alagoas, Brazil. Rev Panam Salud Publica 30, 51–58. [PubMed] [Google Scholar]
- 60. Leal LP, Batista Filho M, Lira PICd et al. (2011) Prevalence of anemia and associated factors in children aged 6–59 months in Pernambuco, Northeastern Brazil. Rev Saúde Pública 45, 457–466. [DOI] [PubMed] [Google Scholar]
- 61. Santos RFd, Gonzalez ESC, Albuquerque ECd et al. (2011) Prevalence of anemia in under 5-year-old children in a children’s hospital in Recife, Brazil. Rev Bras Hematol Hemoter 33, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gondim SSR, Diniz AdS, Souto RAd et al. (2012) Magnitude, time trends and factors associate with anemia in children in the state of Paraíba, Brazil. Rev Saúde Pública 46, 649–656. [DOI] [PubMed] [Google Scholar]
- 63. Pereira JF, Oliveira MAA & Oliveira JS (2012) Anemia in indigenous children of Karapotó ethnic backgrounds. Rev Bras Saude Mater Infant 12, 375–382. [Google Scholar]
- 64. Pedraza DF, Rocha ACD & Sousa CPdC (2013) Growth and micronutrient deficiencies: profile of children attended at the day care center for the government of Paraiba, Brazil. Cien Saude Colet 18, 3379–3390. [DOI] [PubMed] [Google Scholar]
- 65. Cavalcanti DS, Vasconcelos PND, Muniz VM et al. (2014) Iron intake and its association with iron-deficiency anemia in agricultural workers’ families from the Zona da Mata of Pernambuco, Brazil. Rev Nutr 27, 217–227. [Google Scholar]
- 66. Hermes L, Fischer MDQ, Pacheco JP et al. (2014) Presence of anemia, adhesion and time of supplementation with ferrous sulfate in preschools from Venâncio Aires, RS. Rev Jov Pesq 4, 25–34. [Google Scholar]
- 67. Paula WKASd, Caminha MdFC, Figueirôa JN et al. (2014) Anemia and vitamin A deficiency in children under five years old attended by the Family Health Program in the State of Pernambuco, Brazil. Cien Saude Colet 19, 1209–1222. [DOI] [PubMed] [Google Scholar]
- 68. Vasconcelos PNd, Cavalcanti DS, Leal LP et al. (2014) Time trends in anemia and associated factors in two age groups (6–23 and 24–59 months) in Pernambuco State, Brazil, 1997–2006. Cad Saude Publica 30, 1777–1787. [DOI] [PubMed] [Google Scholar]
- 69. Duarte CRdS & de Moura Lima AB (2015) Nutritional status and ferric profile in children attending a school clinic. Rev Interd 8, 107–114. [Google Scholar]
- 70. Ferreira HdS & Torres ZMC (2015) A quilombola community in the Northeast region of Brazil: the health of women and children before and after certification. Rev Bras Saude Mater Infant 15, 219–229. [Google Scholar]
- 71. Matos TA, Arcanjo FPN, Santos PR et al. (2015) Prevention and treatment of anemia in infants through supplementation, assessing the effectiveness of using iron once or twice weekly. J Trop Pediatr 62, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Landim LA (2016) Impact of the two different iron fortified cookies on treatment of anemia in preschool children in Brazil. Nutr Hosp 33, 1013–1256. [DOI] [PubMed] [Google Scholar]
- 73. Pedraza DF (2016) Health and nutrition status of children assisted in public daycare centers of Campina Grande, Paraíba. Cad Saúde Colet 24, 200–208. [Google Scholar]
- 74. Novaes TG, Gomes AT, Silveira KCD et al. (2017) Prevalence and factors associated with anemia in children enrolled in daycare centers: a hierarchical analysis. Rev Paul Pediatr 35, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Magalhães EIdS, Maia DS, Pereira Netto M et al. (2018) Hierarchical analysis of the factors associated with anemia in infants. Rev Paul Pediatr 36, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vieira RCdS, Livramento ARSd, Calheiros MSC et al. (2018) Prevalence and temporal trend (2005–2015) of anaemia among children in Northeast Brazil. Public Health Nutr 21, 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Compri PC, Cury MCFS, Novo NF et al. (2007) Mother and child factors related to occurrence of anemia in children assisted at primary health care centers of São Paulo city, Brazil. Rev Paul Pediatr 25, 349–354. [Google Scholar]
- 78. Konstantyner T, Taddei JAdAC & Palma D (2007) Risk factors for anemia in infants enrolled in public or philanthropic day-care centers in São Paulo city, Brazil. Rev Nutr 20, 349–359. [Google Scholar]
- 79. Modesto SP, Devincenzi MU & Sigulem DM (2007) Feeding practices and nutritional status of children in the second semester of life who receive care in public health facilities. Rev Nutr 20, 405–415. [Google Scholar]
- 80. Nogueira-de-Almeida CA, Ramos APP, João CA et al. (2007) Jardinópolis without anemia, first stage: anthropometric and iron nutrition status evaluation. Rev Paul Pediatr 25, 254–257. [Google Scholar]
- 81. Souto TS, Oliveira MdN, Casoy F et al. (2007) Anemia and per capita income in children enrolled in a childhood education center in São Paulo, Brazil. Rev Paul Pediatr 25, 161–166. [Google Scholar]
- 82. Biscegli TS, Corrêa CEC, Romera J et al. (2008) Nutritional status and iron deficiency among children enrolled in a day care center before and after 15 months of nutritional management. Rev Paul Pediatr 26, 124–129. [Google Scholar]
- 83. Camillo CC, Amancio OMS, Vitalle MSdS et al. (2008) Anemia and nutritional status of children in day-care centers in Guaxupé. Rev Assoc Med Bras 54, 154–159. [DOI] [PubMed] [Google Scholar]
- 84. Carvalho MR, de Souza Chaves T, Lamounier JA et al. (2008) Tendency of the anemia in infants of day care centers of the regional east of Belo Horizonte, MG. Rev Med Minas Gerais 18, S63–S69. [Google Scholar]
- 85. Coutinho GGPL, Goloni-Bertollo EM & Pavarino-Bertelli ÉC (2008) Effectiveness of two programs of intermittent ferrous supplementation for treating iron-deficiency anemia in infants: randomized clinical trial. Sao Paulo Med J 126, 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fujimori E, Duarte LS, Minagawa ÁT et al. (2008) Social reproduction and anemia in infancy. Rev Lat Am Enfermagem 16, 245–251. [DOI] [PubMed] [Google Scholar]
- 87. Gomes KO, Cotta RMM, Euclydes MP et al. (2008) Iron-deficiency anemia in a group of children in a rural community in the region of Zona da Mata, state of Minas. Nutrire 36, 83–96. [Google Scholar]
- 88. Oliveira WLd, Oliveira FLC & Amancio OMS (2008) Nutritional status, hematological and serum levels of iron in preschool children from cities with different child development indexes. Rev Paul Pediatr 26, 225–230. [Google Scholar]
- 89. Prieto BP, Goulart RMM, Mendes GAN et al. (2008) Evaluation of the nutritional state and the prevalence of iron-deficiency anemia in children in a day care center in São Paulo district. Rev Bras Cienc Saude 6, 13–20. [Google Scholar]
- 90. Rocha DdS, Lamounier JA, Capanema FD et al. (2008) Nutritional status and anemia prevalence in children enrolled at day care centers in Belo Horizonte, Minas Gerais, Brazil. Rev Paul Pediatr 26, 6–13. [Google Scholar]
- 91. Shibukawa AF, Silva EMKd, Ichiki WA et al. (2008) Prophylaxis for iron deficiency anemia using ferrous sulfate among infants followed up at a primary healthcare unit in the municipality of Embu-SP (2003/2004). Sao Paulo Med J 126, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bagni UV, Baião MR, Santos MMAdS et al. (2009) Effect of weekly rice fortification with iron on anemia prevalence and hemoglobin concentration among children attending public daycare centers in Rio de Janeiro, Brazil. Cad Saude Publica 25, 291–302. [DOI] [PubMed] [Google Scholar]
- 93. Costa C, Machado E, Colli C et al. (2009) Anemia em pré-escolares atendidos em creches de São Paulo (SP): perspectivas decorrentes da fortificação das farinhas de trigo e de milho* (Anemia in pre-school children attending day care centers of São Paulo: perspectives of the wheat). Nutrire 34, 59–74. [Google Scholar]
- 94. Ferreira CDMO (2009) Hematological analysis and biochemical profile of iron and ferritin in children aged 1 to 6 years students of a municipal school in Uberaba – MG, from August to November 2008. AC&T Cient 1, 1–16. [Google Scholar]
- 95. Jordão RE, Bernardi JLD & Barros Filho AdA (2009) Feeding pattern and anemia in infants in the city of Campinas, São Paulo, Brazil. Rev Paul Pediatr 27, 381–388. [Google Scholar]
- 96. Konstantyner T, Taddei JA, Oliveira MN et al. (2009) Isolated and combined risks for anemia in children attending the nurseries of daycare centers. J Pediatr 85, 209–216. [DOI] [PubMed] [Google Scholar]
- 97. Sá ACEd & Szarfarc SC (2009) Prevalence of anemia in children, before and during participation in the iron fortification program. Nutrire 34, 115–126. [Google Scholar]
- 98. Reis MCGd, Nakano AMS, Silva IA et al. (2010) Prevalence of anemia in children 3 to 12 months old in a health service in Ribeirão Preto, SP, Brazil. Rev Lat Am Enfermagem 18, 792–799. [DOI] [PubMed] [Google Scholar]
- 99. Righi CGB & de Oliveira CAS (2010) Identification of the prevalence for anemia in children aged 6 to 24 months in the city of Guaruja (SP). Unilus Ens Pesq 7, 15–25. [Google Scholar]
- 100. Santos JN, Lemos SMA, Lamounier JA et al. (2010) Impact of anemia in children’s language development: prospective longitudinal study. Rev Med Minas Gerais 20, 519–527. [Google Scholar]
- 101. Azeredo CM, Cotta RMM, Silva LSd et al. (2011) Implantation and impact of the national program of iron supplementation in the city of Viçosa, MG, Brazil. Cien Saude Colet 16, 4011–4022. [DOI] [PubMed] [Google Scholar]
- 102. Capanema FD, Filho LCC, Pedrosa RM et al. (2011) Accuracy of clinical examination in determing anemia in children. Rev Med Minas Gerais 21, 6–11. [Google Scholar]
- 103. Costa JT, Bracco MM, Gomes PAP et al. (2011) Prevalence of anemia among preschoolers and response to iron supplementation. J Pediatr (Rio J) 87, 76–79. [DOI] [PubMed] [Google Scholar]
- 104. Cotta RMM, Fabiana de Cássia Carvalho O, Magalhães KA et al. (2011) Social and biological determinants of iron deficiency anemia. Cad Saude Publica 27, s309–s320. [DOI] [PubMed] [Google Scholar]
- 105. Netto MP, Silva Rocha Dd, Castro Franceschini SdC et al. (2011) Anemia-associated factors in infants born at term with normal weight. Rev Assoc Med Bras 57, 550–558. [PubMed] [Google Scholar]
- 106. Pessoa MC, Jansen AK, Velásquez-Meléndez G et al. (2011) Factors associated to anemia in infants residents of an urban region. Rev Min Enferm 15, 54–61. [Google Scholar]
- 107. Mendes JCdP, Pandolfi MM, Carabetta V Jr et al. (2012) Factors associated to language disorders in preschool children. Rev Soc Bras Fonoaudiol 17, 177–181. [Google Scholar]
- 108. Netto MP (2012) Iron nutritional status and its association with serum retinol concentration in children. HU Rev 38, 215–221. [Google Scholar]
- 109. Rocha DdS, Capanema FD, Pereira Netto M et al. (2012) Prevalence and risk factors of anemia in children attending daycare centers in Belo Horizonte – MG. Rev Bras Epidemiol 15, 675–684. [DOI] [PubMed] [Google Scholar]
- 110. Castro RGd, Martins-Júnior JA & Lima LM (2013) Risk of iron deficiency anemia in children with low ferritin levels. Infarma 25, 138–142. [Google Scholar]
- 111. Correa MM, Arpini LdSB & Ferreira DM (2013) Nutritional status and prevalence of anemia in children under 36 months. Rev Bras Promoc Saude 27, 109–116. [Google Scholar]
- 112. Coutinho GG, Cury PM & Cordeiro JA (2013) Cyclical iron supplementation to reduce anemia among Brazilian preschoolers: a randomized controlled trial. BMC Public Health 13, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dias ACP & Szarfarc SC (2013) Alternative nutritional intervention to control anemia in children and mothers. Espac Saude 14, 7–13. [Google Scholar]
- 114. Nogueira-de-Almeida CA, De Mello ED, Ramos AP et al. (2013) Assessment of drinking water fortification with iron plus ascorbic acid or ascorbic acid alone in daycare centers as a strategy to control iron-deficiency anemia and iron deficiency: a randomized blind clinical study. J Trop Pediatr 60, 40–46. [DOI] [PubMed] [Google Scholar]
- 115. Oliveira APDNd, Pascoal MN, Santos LCd et al. (2013) The prevalence of anemia and its association with socio-demographic and anthropometric aspects in children living in Vitória, State of Espírito Santo, Brazil. Cien Saude Colet 18, 3273–3280. [PubMed] [Google Scholar]
- 116. Pereira AdS, Peixoto NGdA, Nogueira Neto JF et al. (2013) Nutritional status of childhood of a public day care center: a longitudinal study. Cad Saúde Colet 21, 140–147. [Google Scholar]
- 117. Barreto CTG, Cardoso AM & Coimbra CEA Jr (2014) Nutritional status of Guarani indigenous children in the States of Rio de Janeiro and São Paulo, Brazil. Cad Saude Publica 30, 657–662. [DOI] [PubMed] [Google Scholar]
- 118. Oliveira TdSCd, Silva MCd, Santos JN et al. (2014) Anemia among preschool children – a public health problem in Belo Horizonte, Brazil. Cien Saude Colet 19, 59–66. [DOI] [PubMed] [Google Scholar]
- 119. Saraiva BCA, Soares MCC, Santos LCd et al. (2014) Iron deficiency and anemia are associated with low retinol levels in children aged 1 to 5 years. J Pediatr 90, 593–599. [DOI] [PubMed] [Google Scholar]
- 120. Stulbach TE, Name JJ, Daboin BEG et al. (2014) Efficacy of the national program of iron supplementation in the anaemia control in infants assisted by child education centers. J Hum Growth Dev 24, 282–288. [Google Scholar]
- 121. Clarke SL, Zanin FHC, da Silva CAM et al. (2015) Determinants of iron deficiency anemia in a cohort of children aged 6–71 months living in the Northeast of Minas Gerais, Brazil. PLoS One 10, e0139555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lisbôa MBMdC, Oliveira EO, Lamounier JA et al. (2015) Prevalence of iron-deficiency anemia in children aged less than 60 months: a population-based study from the state of Minas Gerais, Brazil. Rev Nutr 28, 121–131. [Google Scholar]
- 123. Silva MA, Carvalho CAd, Fonsêca PCdA et al. (2015) Prevalence and factors associated with anemia and iron deficiency in children aged 18 to 24 months. Cad Saúde Colet 23, 362–367. [Google Scholar]
- 124. Vaz-Tostes MdG (2015) Nutritional status relative to iron, zinc and vitamin A to preschool children enrolled in a program of food and nutrition education. HU Rev 41, 163–170. [Google Scholar]
- 125. Fonseca CRB, Machado BL & Alquati LR (2016) Anemia and nutritional status of preschool children: comparison between two childhood education centers in Botucatu City, Brazil. Epidemiology 6, e1000282. [Google Scholar]
- 126. Freitas BA, Lima LM, Moreira ME et al. (2016) Micronutrient supplementation adherence and influence on the prevalences of anemia and iron, zinc and vitamin A deficiencies in preemies with a corrected age of 6 months. Clinics 71, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. André HP, Vieira SA, Franceschini SdCC et al. (2017) Factors associated with the iron nutritional status of Brazilian children aged 4 to 7 years. Rev Nutr 30, 345–355. [Google Scholar]
- 128. Lucia CMD, Santos LLM, Anunciação PC et al. (2017) Socioeconomic profile and health conditions of preschoolers from two philanthropic day care centers in the city of Viçosa, MG. Rasbran 8, 3–11. [Google Scholar]
- 129. Man MB, Saito TT, Lambert L et al. (2017) Hemoglobin levels in daycares registered in the health program at the school of city of Santos. Unilus Ens Pesq 14, 157–165. [Google Scholar]
- 130. Mendes GM, Silqueira LA, Paula LP et al. (2017) Anemia among premature infants in the first year. Resid Pediátr 9, 97–103. [Google Scholar]
- 131. Nobre LN, Lessa AdC, Oliveira HCd et al. (2017) Iron-deficiency anemia and associated factors among preschool children in Diamantina, Minas Gerais, Brazil. Rev Nutr 30, 185–196. [Google Scholar]
- 132. Silva MC, Capanema FD, Oliveira TdSC et al. (2019) Temporal trend of anemia in children from public day care centers in Belo Horizonte – MG. Percurso Academico 9, 310–328. [Google Scholar]
- 133. Siqueira MCG, Bezerra LdS, Freitas CZGd et al. (2019) Association of nutritional status with markers of iron deficiency anemia in preschoolers attended at a family health unity of Presidente Prudente-SP. Colloquium Vitae 12, 8–19. [Google Scholar]
- 134. Rocha EMB, Lopes AF, Pereira SM et al. (2020) Iron deficiency anemia and its relationship with socioeconomic vulnerability. Rev Paul Pediatr 38, e2019031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Assunção MCF, Santos IdSd, Barros AJDd et al. (2007) Anemia in children under six: population-based study in Pelotas, Southern Brazil. Rev Saúde Pública 41, 328–335. [DOI] [PubMed] [Google Scholar]
- 136. Vitolo MR & Bortolini GA (2007) Iron bioavailability as a protective factor against anemia among children aged 12 to 16 months. J Pediatr (Rio J) 83, 33–38. [DOI] [PubMed] [Google Scholar]
- 137. Martins M & Cornbluth S (2008) Risk factors for iron deficiency in infants assisted in the clinic of a university hospital: nutritional education and prevention of anemia. Nutrire 33, 49–60. [Google Scholar]
- 138. Fontoura S, Coser J, Fontoura T et al. (2009) Prevalence of anemia in children from 1 to 5 years old inhabithantes of the Passo district, Arneldo Matter neighbourhood – São Borja/RS and its relationship with nutritional condition and enteroparasitosis. Rev Bras Anal Clin 41, 103–108. [Google Scholar]
- 139. Pazzinato M & Herrero JCM (2009) Prevalence of anemia and associated factors in patients from Luiziana city of Paraná. Rev Bras Anal Clin 41, 151–154. [Google Scholar]
- 140. Bortolini GA & Vitolo MR (2010) Relationship between iron deficiency and anemia in children younger than 4 years. J Pediatr 86, 488–492. [DOI] [PubMed] [Google Scholar]
- 141. Pan MS, Liz C, Franco SC et al. (2011) Breastfeeding, anemia, and nutritional status of children between 6 and 12 months of age monitored in Family Health Units. Saúde Debate 35, 73–82. [Google Scholar]
- 142. Rodrigues VC, Mendes BD, Gozzi A et al. (2011) Iron deficiency and prevalence of anemia and associated factors in children attending public daycare centers in western Paraná, Brazil. Rev Nutr 24, 407–420. [Google Scholar]
- 143. Scherer F & Beneduzi VL (2011) Nutritional profile and prevalence of iron deficiency anemia in children. ConScientiae Saúde 10, 433–440. [Google Scholar]
- 144. Silva EBd, Villani MS, Jahn AdC et al. (2011) Risk factors associated with iron deficiency anaemia in children from 0 to 5 year old in a municipality to the northeast region of Rio Grande do Sul. Rev Min Enferm 15, 165–173. [Google Scholar]
- 145. Bortolini GA & Vitolo MR (2012) The impact of systematic dietary counseling during the first year of life on prevalence rates of anemia and iron deficiency at 12–16 months. J Pediatr (Rio J) 88, 33–39. [DOI] [PubMed] [Google Scholar]
- 146. Hintz RS & Teixeira ML (2012) Prevalence of anemia in 6-month- to 6-year-old children attended at the clinical analyses laboratory. Saúde Pesq 5, 87–95. [Google Scholar]
- 147. Pacheco JP, Silva Schedler FL, Hermes L et al. (2013) Prevalence of anemia and associated factors in children aged 6 to 24 months enrolled in the public school system of Venâncio Aires, RS, Brazil. Rev Jov Pesq 3, 179–190. [Google Scholar]
- 148. Silla LM, Zelmanowicz A, Mito I et al. (2013) High prevalence of anemia in children and adult women in an urban population in southern Brazil. PLoS One 8, e68805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zuffo CR, Osorio MM, Taconeli CA et al. (2016) Prevalence and risk factors of anemia in children. J Pediatr 92, 353–360. [DOI] [PubMed] [Google Scholar]
- 150. Cembranel F, Corso ACT & González-Chica DA (2017) Inadequacies in the treatment of iron deficiency anemia among children registered in the National Program of Iron Supplementation in Florianopolis, Santa Catarina, Brazil. Texto Contexto Enferm 26, e06310015. [Google Scholar]
- 151. Fischer MdQ, Molz P, Hermes L et al. (2017) Neuropsychomotor development and genomic stability associated to folate and blood iron levels in preschool children. Rev Bras Saude Mater Infant 17, 511–518. [Google Scholar]
- 152. Rodrigues VB, Dallazen C & Vítolo MR (2017) Impact of health professionals training in infant feeding practices on the prevalence of anemia in children: randomized field trial. Rev Inova Saúde 6, 1–19. [Google Scholar]
- 153. Melere C & De Oliveira TM (2018) Contribution of early weaning in the occurrence of iron-deficiency anemia in infants. Arch Health Sci 25, 32–35. [Google Scholar]
- 154. IBGE (2010) Pesquisa de Orçamentos Familiares 2008–2009. Anthropometry and Nutritional Status of Children, Adolescents and Adults in Brazil. Rio de Janeiro: Ministry of Health, IBGE 64.03:001.8-P474p. [Google Scholar]
- 155. Brasil (2009) National Survey of Demography and Health of Children and Women-PNDS 2006: Dimensions of the Reproductive Process and Child Health. Brasília, Brazil: Ministério da Saúde. [Google Scholar]
- 156. da Silva Pereira A, de Castro IRR, Bezerra FF et al. (2020) Reproducibility and validity of portable haemoglobinometer for the diagnosis of anaemia in children under the age of 5 years. J Nutr Sci 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Konstantyner T, Roma Oliveira TC & de Aguiar Carrazedo Taddei JA (2012) Risk factors for anemia among Brazilian infants from the 2006 National Demographic Health Survey. Anemia 2012, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vieira RCS & Ferreira HS (2010) Prevalence of anemia in Brazilian children in different epidemiological scenarios. Rev Nutr 23, 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Dutra-de-Oliveira JE, Lamounier JA, Nogueira-de-Almeida CA et al. (2007) Fortification of drinking water to control iron-deficiency anemia in preschool children. Food Nutr Bull 28, 173–180. [DOI] [PubMed] [Google Scholar]
- 160. Dutra-de-Oliveira JE, Marchini JS, Lamounier J et al. (2011) Iron-fortified drinking water studies for the prevention of children’s anemia in developing countries. Anemia 2011, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Brasil (2002) Wheat flour and/or iron-fortified corn. In Resoluçåo RDC No 344, vol. 344, pp. 1–3 [Saúde Md, editor]. Brasília: Ministério da Saúde do Brasil. [Google Scholar]
- 162. Brasil (2005) Módulo de gerenciamento do programa. In Operational Manual of the National Iron Supplementation Program, pp. 28 [Básica DdA, editor]. Brasília: Ministério da Saúde Brasília. [Google Scholar]
- 163. Lozoff B (2007) Iron deficiency and child development. Food Nutr Bull 28, S560–S571. [DOI] [PubMed] [Google Scholar]
- 164. Lozoff B (2011) Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 141, 740S–746S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lozoff B, Beard J, Connor J et al. (2006) Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64, S34–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Borges CVD, Veiga APB, Barroso GdS et al. (2007) Association among serum concentration of minerals, anthropometric indices and diarrhea in low-income children in the metropolitan region of Rio de Janeiro, Brazil. Rev Nutr 20, 159–169. [Google Scholar]
- 167. Labib AG, El-Bana SM, Ahmed SM et al. (2018) The effect of chronic anemia on physical growth and development among children under 5 years. Minia Sci Nutr J 4, 11–21. [Google Scholar]
- 168. Pedraza DF, Rocha ACD & Sales MC (2013) Micronutrient deficiencies and linear growth: a systematic review of observational studies. Cien Saude Colet 18, 3333–3347. [DOI] [PubMed] [Google Scholar]
- 169. Zimmermann MB (2006) The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr 26, 367–389. [DOI] [PubMed] [Google Scholar]
- 170. Pivina L, Semenova Y, Doşa MD et al. (2019) Iron deficiency, cognitive functions, and neurobehavioral disorders in children. J Mol Neurosci 68, 1–10. [DOI] [PubMed] [Google Scholar]
- 171. Christian P (2010) Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 304, 2716–2723. [DOI] [PubMed] [Google Scholar]
- 172. Pala E, Erguven M, Guven S et al. (2010) Psychomotor development in children with iron deficiency and iron-deficiency anemia. Food Nutr Bull 31, 431–435. [DOI] [PubMed] [Google Scholar]
- 173. Tam E, Keats EC, Rind F et al. (2020) Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low- and middle-income countries: a systematic review and meta-analysis. Nutrients 12, 2–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hassan TH, Badr MA, Karam NA et al. (2016) Impact of iron deficiency anemia on the function of the immune system in children. Medicine 95, e5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gombart AF, Pierre A & Maggini S (2020) A review of micronutrients and the immune system – working in harmony to reduce the risk of infection. Nutrients 12, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. de Pontual L (2017) Iron and susceptibility to infections. Arch Pediatr 24, 5S14–5S17. [DOI] [PubMed] [Google Scholar]
- 177. Martins AC, Almeida JI, Lima IS et al. (2017) Iron metabolism and the inflammatory response. IUBMB Life 69, 442–450. [DOI] [PubMed] [Google Scholar]
- 178. Nairz M, Dichtl S, Schroll A et al. (2018) Iron and innate antimicrobial immunity—depriving the pathogen, defending the host. J Trace Elem Med Biol 48, 118–133. [DOI] [PubMed] [Google Scholar]
- 179. Armitage AE & Moretti D (2019) The importance of iron status for young children in low- and middle-income countries: a narrative review. Pharmaceuticals 12, ph12020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jayaweera JAAS, Reyes M & Joseph A (2019) Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep 9, 12637. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181. Bortolini GA, Vitolo MR, Gubert MB et al. (2013) Early cow’s milk consumption among Brazilian children: results of a National Survey. J Pediatr 89, 608–613. [DOI] [PubMed] [Google Scholar]
- 182. Griebler U, Bruckmuller MU, Kien C et al. (2016) Health effects of cow’s milk consumption in infants up to 3 years of age: a systematic review and meta-analysis. Public Health Nutr 19, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Oliveira MA & Osorio MM (2005) Cow’s milk consumption and iron deficiency anemia in children. J Pediatr 81, 361–367. [DOI] [PubMed] [Google Scholar]
- 184. Bezerra AGN, Leal VS, Lira PICd et al. (2018) Anemia and associated factors in women at reproductive age in a Brazilian Northeastern municipality. Rev Bras Epidemiol 21, e180001. [DOI] [PubMed] [Google Scholar]
- 185. Bortolini GA & Vitolo MR (2010) Importance of food practices during the first year of life to prevent iron deficiency. Rev Nutr 23, 1051–1062. [Google Scholar]
- 186. Gaitán CD, Olivares GM, Arredondo OM et al. (2006) Bioavailability of iron in humans. Rev Child Nutr 33, 142–148. [Google Scholar]
- 187. Mantadakis E, Chatzimichael E & Zikidou P (2020) Iron deficiency anemia in children residing in high and low-income countries: risk factors, prevention, diagnosis and therapy. Mediterr J Hematol Infect Dis 12, e2020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. IBGE (2020) Family Budget Survey: 2017–2018: Food Security Analysis in Brazil. Brasília: Instituto Brasileiro de Geografia e Estatística. [Google Scholar]
- 189. Brito LL, Blanton RE, Parraga IM et al. (2006) Moderate- and low-intensity co-infections by intestinal Helminths and Schistosoma Mansoni, dietary iron intake, and anemia in Brazilian children. Am J Trop Med Hyg 75, 939–944. [PubMed] [Google Scholar]
- 190. IPEA (2016) Human Development in Brazilian Macro-Regions, 1st ed., vol. 1, PNUD-IPEA. Brasília: IPEA. [Google Scholar]
- 191. IBGE (2020) National Basic Sanitation Survey, Population Coordination and Social Indicators. Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística. [Google Scholar]
- 192. Sterne JAC, Sutton AJ, Ioannidis JPA et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100286X.
click here to view supplementary material