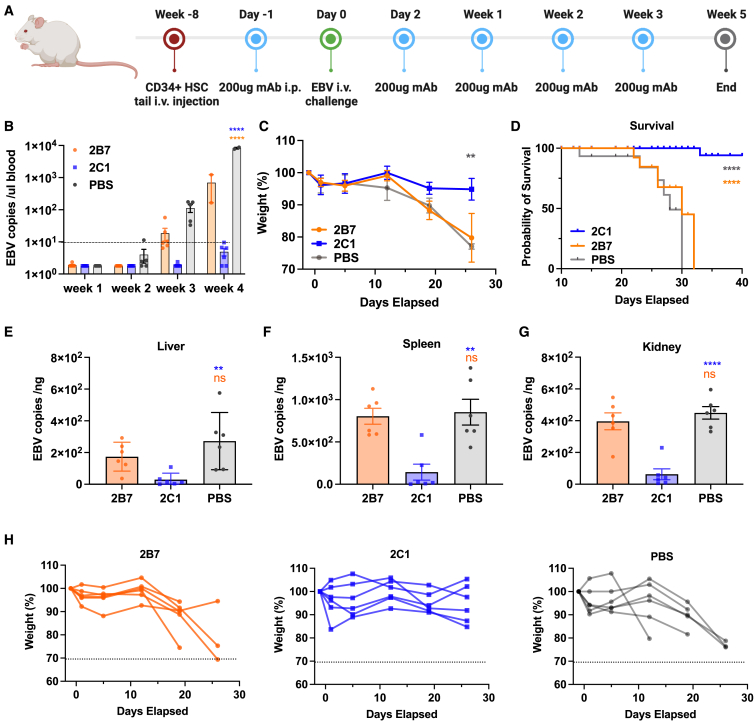
Figure 5.
2C1 provides effective protection against EBV infection in humanized mice
(A) Timeline represents the engraftment of CD34+ human hematopoietic stem cells, the administration of antibodies, and the EBV challenge. Intraperitoneal administration of 200 μg 2C1 (n = 6), 200 μg 2B7 (n = 6), or 200 μL PBS (negative control, n = 6) was performed in mice for a total of five doses. Following the initial dose, the mice were intravenously challenged with CNE2-EBV.
(B) EBV DNA copies in the peripheral blood of each group of mice following the EBV infection. Each data point represents an individual mouse, with the dotted line indicating the detection limit.
(C) The body weight changes of mice in the 2C1, 2B7, and PBS groups were monitored.
(D) The survival curve illustrates the outcomes of mice from each group following the EBV challenge.
(E–G) EBV DNA copies in the liver (E), spleen (F), and kidney (G) were quantified using real-time qPCR.
(H) The body weight of each mouse in the 2B7, 2C1, or PBS group is indicated.
Significance was calculated using two-way ANOVA followed by Dunnett’s multiple comparisons (B and C), log-rank test (D), or one-way ANOVA followed by Tukey’s multiple comparisons (E–G). Error bars, mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.