Abstract
Practical relevance:
Uroliths occur commonly in the bladder and/or urethra of cats and can be lifethreatening if urethral obstruction occurs. Calcium oxalate accounts for 40–50% of urocystoliths and these stones are not amenable to medical dissolution; therefore, removal by surgery or minimally invasive techniques is required if uroliths must be treated. Medical protocols for prevention involve decreasing urine saturation for minerals that form uroliths.
Etiopathogenesis:
Formation of uroliths is not a disease, but rather a complication of several disorders. Some disorders can be identified and corrected (such as infection-induced struvite urolith formation); others can be identified but not corrected (such as idiopathic hypercalcemia). In most cats with calcium oxalate urolith formation the underlying etiopathogenesis is not known. A common denominator of all these disorders is that they can from time to time create oversaturation of urine with one or more crystal precursors, resulting in formation of crystals.
Basic concepts:
In order to develop rational and effective approaches to treatment, abnormalities that promote urolith formation must be identified, with the goal of eliminating or modifying them. It is important, therefore, to understand several basic concepts associated with urolithiasis and the factors that promote urolith formation that may be modified with medical treatment; for example, the state of urinary saturation, modifiers of crystal formation, potential for multiple crystal types, and presence of bacterial infection or urinary obstruction.
Diagnosis of urolithiasis
Imaging
Imaging is the most definitive diagnostic tool for detection of uroliths. Abdominal radiography, for detection of radiopaque uroliths, is generally the first diagnostic imaging modality used (Figure 1). Ultrasonography (Figure 2) or double contrast cystography can be used to detect uroliths (including those that are radiolucent). 2 As well as detecting the presence of uroliths, abdominal imaging is used to verify their location, number, size, shape and density.
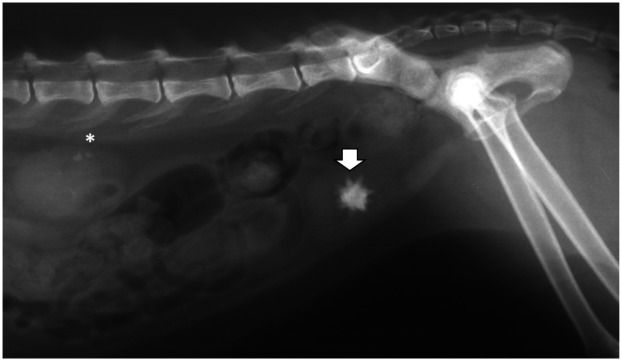
Figure 1.
Lateral abdominal radiograph of an 8-year-old, castrated male domestic shorthair cat showing one calcium oxalate dihydrate urocystolith (arrow). Renal mineralization is also present (asterisk)
Figure 2.
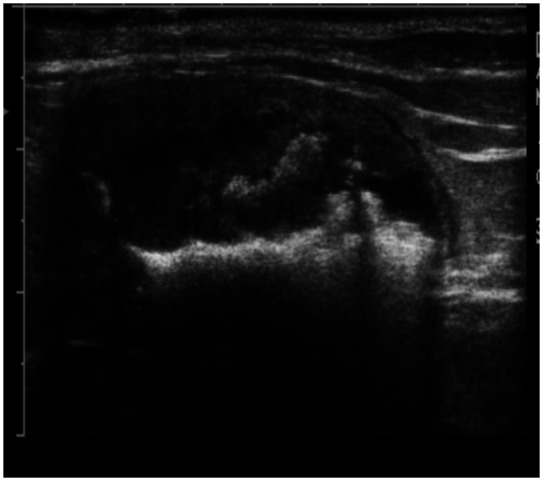
Ultrasonographic image of the urinary bladder of a 14-year-old, castrated male domestic shorthair cat with urolithiasis. Urocystoliths appear as shadowing hyperechoic structures

Urinalysis
In patients with suspected urinary tract disorders, urinalysis is a valuable part of the diagnostic evaluation. Crystalluria can be an important finding (Figure 3). Crystals do not confirm the presence of uroliths but they do suggest crystalline oversaturation; some patients may have active urocystoliths present but not have crystalluria. 3 Temperature changes with time elapsed between urine collection and urinalysis can cause crystals to form in urine, resulting in a false positive crystalluria. 4 Therefore, in patients with suspected urolithiasis, freshly collected urine should always be evaluated. 5
Figure 3.
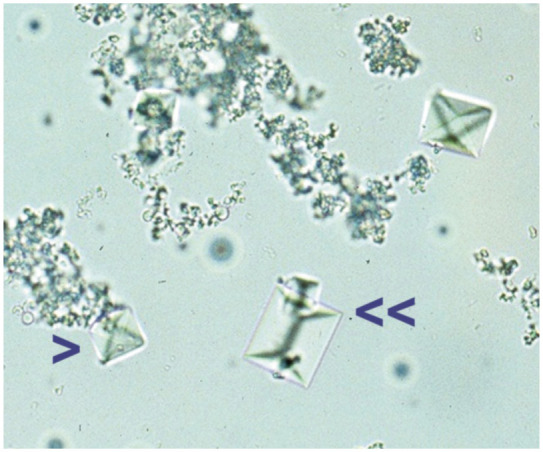
Crystalluria: struvite (double arrow) and calcium oxalate (single arrow) crystals in a urine sample collected from a 6-year-old, castrated male domestic shorthair cat
Urine specific gravity and urine pH can help assess the chemical environment of the urine and, in turn, give an indication of which type of urolith is present. A high urine specific gravity suggests an increase in concentration of urolithic precursors. 6 Calcium oxalate uroliths form typically in urine with a pH less than 6.8. 5
Urine culture and sensitivity testing is indicated because urinary tract infections may occur secondarily in patients with calcium oxalate urolithiasis.7–9 Factors contributing to this include mucosal damage induced by the stones, incomplete urine voiding or microorganism entrapment in the stones.
Clinical biochemistry
When uroliths are found, it is important to obtain a blood biochemical profile, as results can sometimes suggest the presence of underlying disorders such as hypercalcemia that can predispose patients to calcium oxalate urolith formation.10–13 Because uroliths occasionally cause obstruction, electrolyte, mineral, creatinine and blood urea nitrogen concentrations should be monitored as needed.14,15
Risk factors for calcium oxalate crystal and urolith formation
Determining the composition of uroliths is essential to prevent recurrence (see box below).
Calcium oxalate accounts for 40–50% of all uroliths in cats. Risk factors for calcium oxalate formation include increased urinary calcium and/or oxalate excretion and aciduria. Certain breeds of cats are predisposed to calcium oxalate urolith formation including longhaired cats (Burmese, Persian and Himalayan breeds).17–19 Calcium oxalate urolith formation occurs when urine is oversaturated with calcium and oxalate. 1 In addition to these alterations in activities of ions, large molecular weight proteins occurring in urine, such as nephrocalcin, uropontin and Tamm-Horsfall glycoprotein, influence calcium oxalate formation. 20 We have limited understanding of the role of these macromolecular and ionic inhibitors of calcium oxalate formation in cats.
Certain metabolic factors are known to increase the risk of calcium oxalate urolith formation in several species, including cats, as discussed below. Medical and nutritional strategies for stone prevention have focused on amelioration of these factors (see later).
Calcium
Calcium homeostasis is achieved through the actions of parathyroid hormone (PTH) and 1,25-dihydroxycholecalciferol (1,25-vitamin D) on bones, intestines and kidneys, as discussed in a recent review. 21 Briefly, when serum ionized calcium concentration decreases, PTH and 1,25-vitamin D activities increase, resulting in mobilization of calcium from bone, increased absorption of calcium from the intestine, and increased reabsorption of calcium by renal tubules. Conversely, high serum ionized calcium concentration suppresses release of PTH and production of 1,25-vitamin D, resulting in decreased bone mobilization, decreased intestinal absorption of calcium, and increased urinary excretion of calcium. Therefore, hypercalciuria can result from hypercalcemia, excessive intestinal absorption of calcium (gastrointestinal hyperabsorption), impaired renal reabsorption of calcium (renal leak) and/or excessive skeletal mobilization of calcium (resorptive).
Hypercalcemia is associated with increased risk of calcium oxalate urolith formation. In cats with calcium oxalate uroliths, hypercalcemia was observed in 35% of cases. 22 Conversely, uroliths developed in 35% of cats with idiopathic hypercalcemia. 12 Hypercalcemia results in increased calcium fractional excretion and hypercalciuria when severe.
In humans and dogs, as well as in cats, hypercalciuria is a significant risk factor, but not necessarily the cause of calcium oxalate urolith formation. 23 Hypercalciuria has not been well defined in normocalcemic cats with calcium oxalate uroliths, but is thought to occur. Although excessive dietary intake of calcium may theoretically result in hypercalciuria in cats, studies in humans refute this. Apparently, dietary calcium may bind to dietary oxalic acid, resulting in calcium oxalate formation in the lumen of the gastrointestinal tract and thereby preventing absorption of calcium and oxalate. Hypercalciuria may also be a sequela to administration of loop diuretics, glucocorticoids, urinary acidifiers, and vitamin D and/or C.
Oxalic acid
Oxalic acid is a metabolic end product of ascorbic acid (vitamin C) and several amino acids, such as glycine and serine, derived from dietary sources. Oxalic acid forms soluble salts with sodium and potassium ions, but a relatively insoluble salt with calcium ions. Therefore, any increase in urinary concentration of oxalic acid may promote calcium oxalate formation. Dietary increases of oxalate and vitamin B6 deficiency are known factors increasing urinary oxalate. Hyperoxaluria has been observed experimentally in kittens consuming vitamin B6 deficient diets, 11 but has not been associated with naturally occurring calcium oxalate urolith formation in adults.
Genetic anomalies may also increase urinary oxalic acid concentration. Hyperoxaluria has been recognized in a group of related cats with reduced quantities of hepatic D-glycerate dehydrogenase, an enzyme involved in metabolism of oxalic acid precursors (primary hyperoxaluria type 2). 24 In humans, hyperoxaluria has also been associated with defective peroxisomal alanine:glyoxylate aminotransferase activity (primary hyperoxaluria type 1) and intestinal disease (enteric hyperoxaluria). It has not been evaluated whether similar occurs in cats (or dogs).
Oxalobacter formigenes is an enteric bacterium that metabolizes oxalic acid in the gastrointestinal tract. It has been shown that dogs which have less enteric colonization with O formigenes have a higher risk of calcium oxalate urolith formation than dogs that are more highly colonized. 25
Compared with humans, urinary oxalate appears to play a lesser role in calcium oxalate formation in cats (and dogs), and urinary calcium appears to play a greater role.26,27
Urine pH
Metabolic acidosis promotes hypercalciuria by promoting bone turnover (release of calcium with buffers from bone), increasing serum ionized calcium concentration and resulting in increased urinary calcium excretion and decreased renal tubular reabsorption of calcium. Consumption of diets supplemented with the urinary acidifier ammonium chloride has been associated with increased urinary calcium excretion in cats. 28 Additionally, in people, consumption of diets containing high amounts of animal protein results in metabolic acidosis and increased urinary calcium excretion.
Low urine pH alters function and concentration of crystal inhibitors. For example, it decreases urinary citrate concentration by increasing renal proximal tubular citrate reabsorption. Acidic urine is also known to impair function of macromolecular protein inhibitors. In dogs, hypercalciuria resulting from ammonium chloride administration was decreased by bicarbonate administration. 29 In cats, magnesium supplementation as magnesium chloride was associated with increased urinary calcium excretion and aciduria, while magnesium supplementation as magnesium oxide was associated with alkaluria and a lesser degree of urinary calcium excretion. 30
Urine pH has a direct effect on solubility of calcium oxalate, albeit a relatively small influence. In a study of healthy cats fed similar diets (differing only in their acidifying or alkalinizing properties), urinary saturation with calcium oxalate was lower when the urine pH was >7.2 and higher when urine pH was <6.5. 31
Urinary inhibitors
Inhibitors, such as citrate, magnesium and pyrophosphate, form soluble salts with calcium or oxalic acid, thus reducing their availability for precipitation. Other inhibitors, such as Tamm-Horsfall glycoprotein and nephrocalcin, interfere with the ability of calcium and oxalic acid to combine, thus minimizing crystal formation, aggregation and growth.
Urine volume
Decreased urine volume results in increased calcium and oxalic acid saturation and a higher risk of urolith formation. Cats can achieve urine specific gravities in excess of 1.065, indicating a marked ability to produce concentrated urine. Many cats affected with calcium oxalate uroliths have a urine specific gravity >1.040 unless there is some impairment of renal function or concentrating ability. 23 Detection of calcium oxalate crystals indicates that urine is supersaturated with calcium oxalate, and, if persistent, represents an increased risk for calcium oxalate urolith formation. However, calcium oxalate crystalluria is present in fewer than 50% of feline (and canine) cases at the time of diagnosis of urolithiasis. 23
Management of urocystoliths
Uroliths may result in clinical signs of lower urinary tract disease including urethral obstruction. Often urethral obstruction is associated with azotemia, hyperkalemia, metabolic acidosis and dehydration. 32 Treatment for urethral obstruction involves relieving the obstruction and correcting the metabolic imbalances as quickly as possible.
Medical protocols that will promote dissolution of calcium oxalate uroliths are not currently available. Therefore, when intervention is indicated (see box), uroliths must be removed physically, either surgically or using a minimally invasive technique such as voiding urohydropropulsion. 33
Surgery
Traditional open surgery remains an option for treatment of urolithiasis. For example, if urethral obstructions are recurrent, perineal urethrostomy in male cats (scrotal urethrostomy in male dogs) may be considered. However, these procedures are associated with increased risk of lower urinary tract disease and bacterial urinary tract infections. 34
Minimally invasive techniques
There are several minimally invasive treatment approaches for retrieval of bladder and urethral stones. These include voiding urohydropropulsion, transurethral cystoscopic stone removal, with or without use of laser lithotripsy, and percutaneous cystolithotomy (also called minilaparotomy-assisted cystoscopic stone removal).
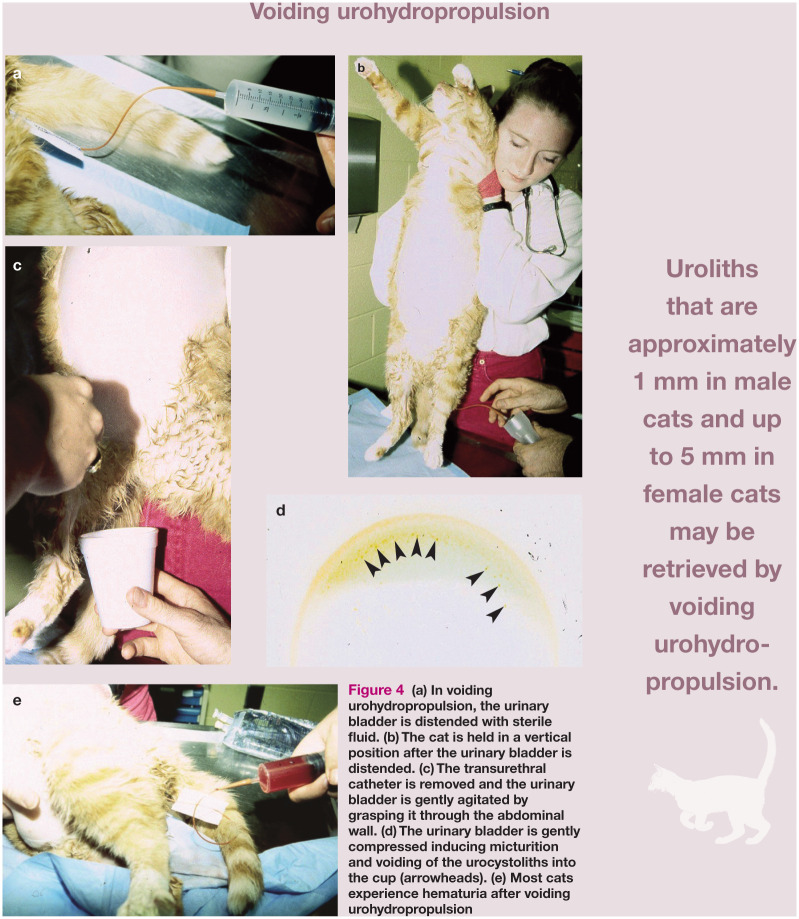
• Catheter-assisted retrieval or voiding urohydropropulsion In catheter-assisted retrieval or voiding urohydropropulsion of calculi, the patient is sedated or anesthetized, a catheter is passed into the urinary bladder transurethrally and the bladder is filled with sterile crystalloid solution. 35 In cats, a 3.5 French or 5 French catheter is used. During catheter retrieval, the contents of the bladder are aspirated while the bladder is agitated by palpating and manipulating it or rotating the patient’s body. This procedure is difficult in most male cats due to the size of the urethra limiting the size of catheter that can be used. With voiding urohydropropulsion, the patient is held vertically while the distended bladder is manually expressed after removing the catheter (Figure 4). 35 Sizes of uroliths that may be retrieved with this technique are approximately 1 mm in male cats and up to 5 mm in female cats.
Figure 4.

(a) In voiding urohydropropulsion, the urinary bladder is distended with sterile fluid. (b) The cat is held in a vertical position after the urinary bladder is distended. (c) The transurethral catheter is removed and the urinary bladder is gently agitated by grasping it through the abdominal wall. (d) The urinary bladder is gently compressed inducing micturition and voiding of the urocystoliths into the cup (arrowheads). (e) Most cats experience hematuria after voiding urohydropropulsion
Thus, these methods are used to eliminate small calculi and to collect them for analysis to plan further treatment. These techniques will not be successful, however, if a patient presents with urethral obstruction as this situation indicates that there is at least one urolith that is too large to pass through the urethra.
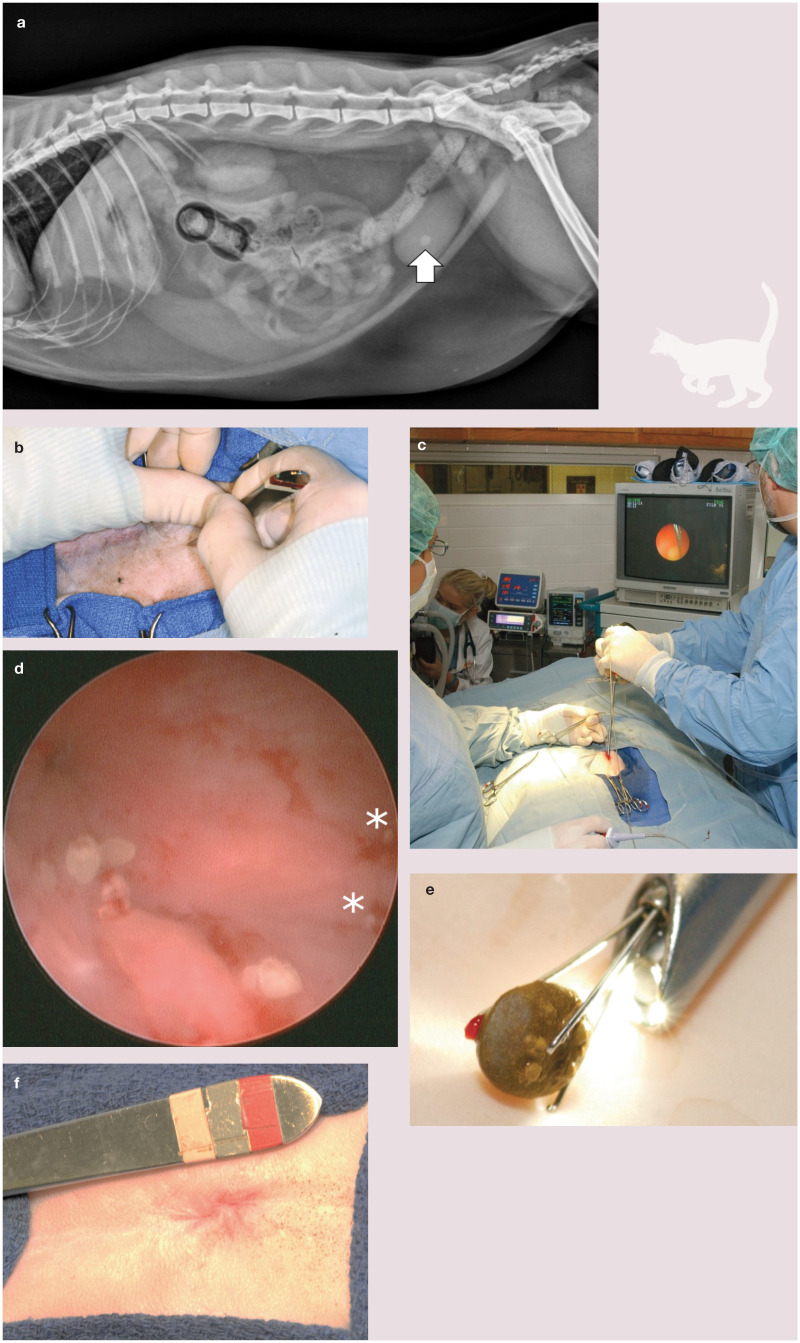
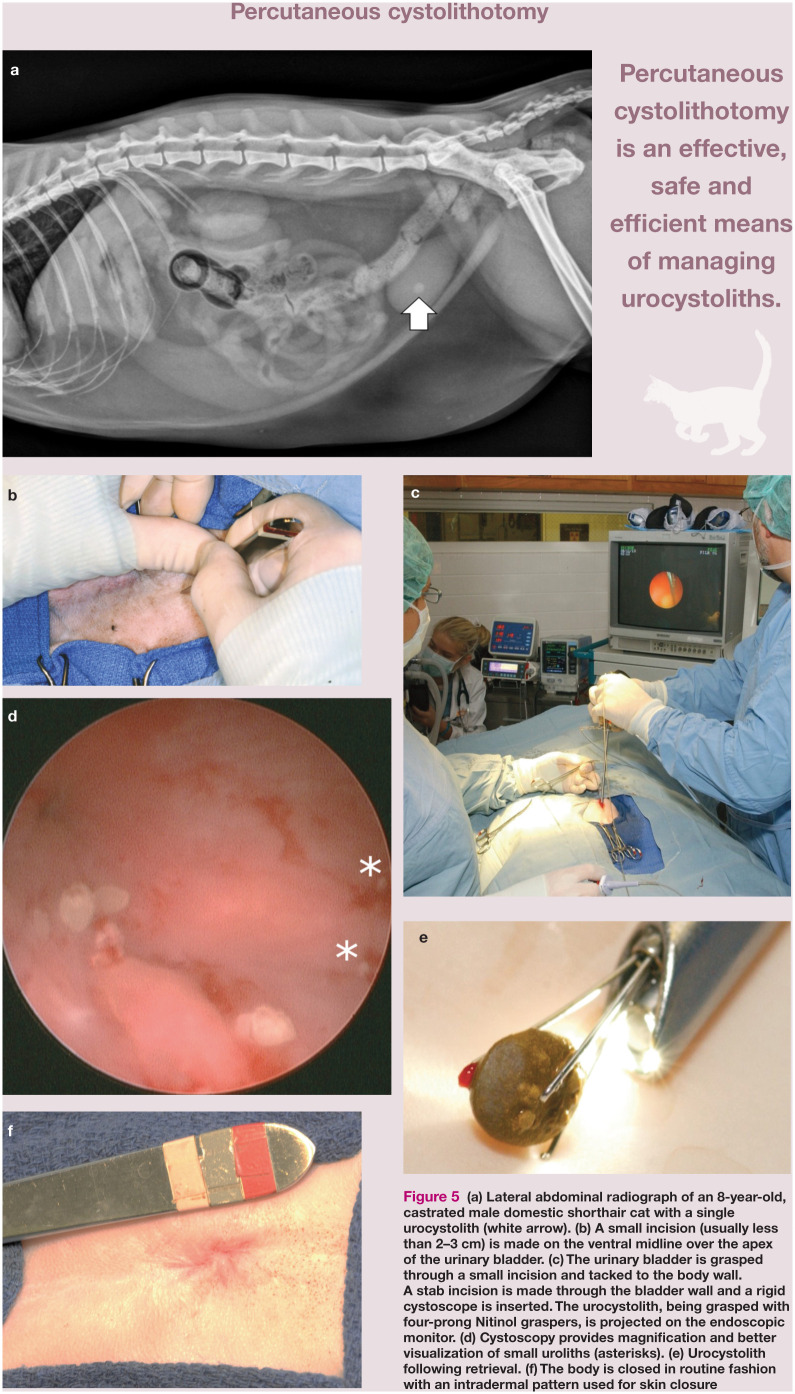
• Percutaneous cystolithotomy This is a procedure where the bladder is temporarily fastened to the incised linea and allows for cystoscopic stone removal through a stab incision or a laparoscopic port placed in the urinary bladder (Figure 5). 36 This method is an effective, safe and efficient means of managing urocystoliths. Cystoscopy produces magnified images of the fluid-distended urinary bladder, allowing identification of abnormalities such as strictures, masses and calculi. 37 This is the minimally invasive procedure of choice for male cats because the diameter of the urethra limits insertion of a cystoscope with an operating channel. Cystoscopic techniques are more efficient than surgical procedures, decreasing the risk of trauma and abdominal contamination. 37
Figure 5.
(a) Lateral abdominal radiograph of an 8-year-old, castrated male domestic shorthair cat with a single urocystolith (white arrow). (b) A small incision (usually less than 2–3 cm) is made on the ventral midline over the apex of the urinary bladder. (c) The urinary bladder is grasped through a small incision and tacked to the body wall. A stab incision is made through the bladder wall and a rigid cystoscope is inserted. The urocystolith, being grasped with four-prong Nitinol graspers, is projected on the endoscopic monitor. (d) Cystoscopy provides magnification and better visualization of small uroliths (asterisks). (e) Urocystolith following retrieval. (f) The body is closed in routine fashion with an intradermal pattern used for skin closure
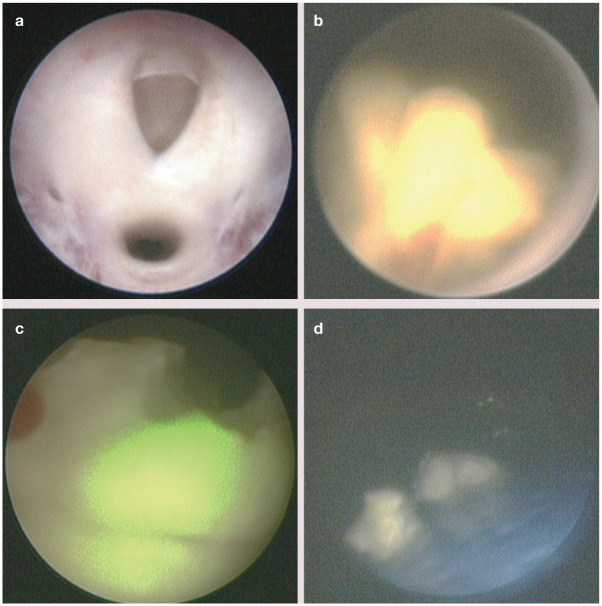
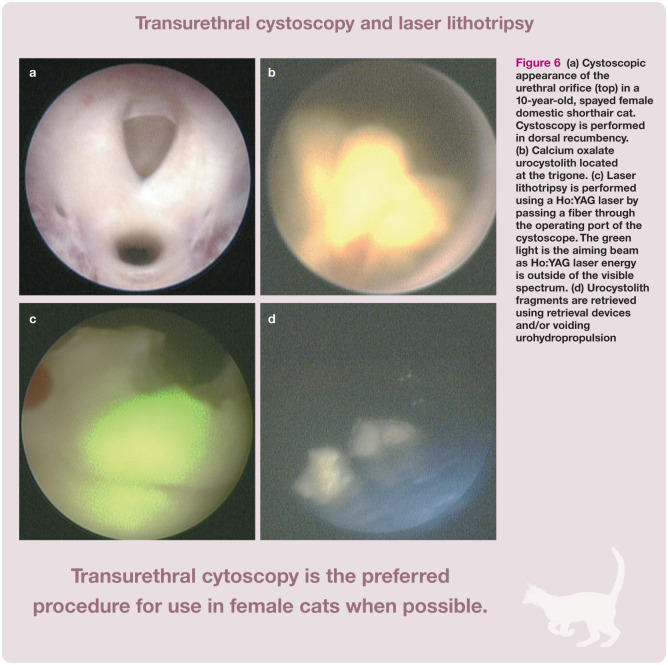
• Transurethral cystoscopy In this technique a cystoscope is inserted into the urethra and passed into the urinary bladder (Figure 6). This is the preferred procedure for use in females because it is less invasive than cystotomy; however, in some female cats percutaneous cystolithotomy may be required due to inability to insert a cystoscope transurethrally. If calculi are small enough, they can be removed using stone retrieval devices such as stone baskets and graspers. For larger calculi, lithotripsy may be used if available. 38 Lithotripsy involves passing a laser fiber through the operating channel of the cystoscope. The fiber emits light at an infrared wavelength causing calculi to fragment;38–43 the resulting fragments are removed transurethrally.
Figure 6.
(a) Cystoscopic appearance of the urethral orifice (top) in a 10-year-old, spayed female domestic shorthair cat. Cystoscopy is performed in dorsal recumbency. (b) Calcium oxalate urocystolith located at the trigone. (c) Laser lithotripsy is performed using a Ho:YAG laser by passing a fiber through the operating port of the cystoscope. The green light is the aiming beam as Ho:YAG laser energy is outside of the visible spectrum. (d) Urocystolith fragments are retrieved using retrieval devices and/or voiding urohydropropulsion

Transurethral cystoscopy is not possible in male cats due to the limiting size of the male cat urethra and inability to insert a large enough scope with an operating channel.
Preventive therapy – dietary strategies
Acidification
Epidemiologic studies consistently identify acidifying diets as being among the most prominent risk factors for calcium oxalate urolithiasis.17,18,45 Solubility of calcium oxalate in urine is minimally influenced by pH; however, there is a linear relationship between increasing calcium oxalate solubility and urine pH in healthy cats, with alkaluria inducing a lower relative supersaturation for calcium oxalate in comparison with aciduria. 31 Persistent aciduria may be associated with low-grade metabolic acidosis, which promotes bone mobilization and increases urinary calcium excretion; however, this effect has not been demonstrated in studies of cats followed for up to 1 year. 31

In a case series of five cats with hypercalcemia and calcium oxalate uroliths, discontinuation of acidifying diets or urinary acidifiers was associated with normalization of serum calcium concentration. 18 Furthermore, aciduria promotes hypocitraturia and functional impairment of endogenous urolith inhibitors. Thus, feeding an acidifying diet or administering urinary acidifiers to cats at risk of calcium oxalate uroliths is contraindicated.
A target urine pH of 6.6–7.5 is suggested in cats at risk of recurrence of calcium oxalate uroliths. 46
Citrate
Potassium citrate is often included in diets designed for calcium oxalate prevention. In urine, citric acid combines with calcium to form soluble complexes, thereby reducing ionized calcium concentration. When oxidized within the tricarboxylic acid cycle, supplemental citrate results in urine alkalinization due to production of bicarbonate. The metabolic alkalinization increases endogenous renal citrate excretion and reduces renal calcium absorption and urinary excretion. 46
Commercial products that add citrate but continue to acidify the urine (pH <6.5) negate the benefit of citrate therapy.

Calcium
Although reduction of urine calcium and oxalic acid concentrations by restriction of dietary calcium and oxalic acid appears logical, it is not without risk. Reducing consumption of only one of these constituents may increase availability and intestinal absorption of the other, resulting in increased urinary excretion. Conversely, increasing dietary calcium levels in normal cats contributes directly to increased urine calcium concentration. Because epidemiologic data in cats suggest that marked dietary calcium restriction increases urolith risk, moderate levels of dietary calcium are advised in non-hypercalcemic cats. 46
Phosphorus
Dietary phosphorus should not be restricted in cats (or dogs) with calcium oxalate urolithiasis. Low dietary phosphorus is a risk factor for calcium oxalate urolith formation in cats. 47 Reduction in dietary phosphorus may be associated with activation of vitamin D, which in turn promotes intestinal calcium absorption and hypercalciuria. Additionally, phosphate status determines pyrophosphate urinary concentrations, an inhibitor of calcium oxalate urolith formation in humans and rodents. If calcium oxalate urolithiasis is associated with hypophosphatemia and normal calcium concentration, oral phosphorus supplementation may be considered. Caution is required, however, because excessive dietary phosphorus may predispose to formation of calcium phosphate uroliths in people. Whether this occurs in cats is unknown.
Magnesium
Urinary magnesium forms complexes with oxalic acid, reducing the amount of oxalic acid available to form calcium oxalate. Studies in cats associate low dietary magnesium with calcium oxalate risk.18,45,47–50 In humans, supplemental magnesium has been used to minimize recurrence of calcium oxalate uroliths; however, supplemental magnesium may increase the risk of struvite formation in cats.
At this time, the risks and benefits of magnesium supplementation in cats with calcium oxalate urolithiasis have not been evaluated and this strategy is not advised. At the same time, it would seem logical that magnesium should not be highly restricted in diets that are consumed by cats with calcium oxalate urolithiasis.
Protein
Consumption of high amounts of animal protein is associated with an increased risk of calcium oxalate formation in people. Dietary protein of animal origin may increase urinary calcium and oxalic acid excretion, decrease urinary citrate excretion, and promote bone mobilization in order to buffer the acid intake from metabolism of animal proteins. Increasing dietary protein from 35–57% (dry matter [DM] basis) increased urine calcium by 35% and decreased urine citrate by 45% in cats. 51 However, a case control study showed that higher protein content in cat foods appeared protective against calcium oxalate uroliths. 47 While several co-associations (eg, higher protein in canned foods) might explain this finding, cats are obligatory carnivores and dietary protein restriction in the management of calcium oxalate urolithiasis is controversial and should be undertaken cautiously.
Vitamins
Excessive intake of vitamin C, a metabolic oxalate precursor, should be avoided. 46 While normal dietary vitamin C levels are not considered a risk in humans, very small increases in urinary oxalate are a concern in urolith formers. Because cats (and dogs) do not have a dietary vitamin C requirement, supplementation should be avoided in foods fed to those at risk of calcium oxalate uroliths. Cranberry concentrate tablets are also contraindicated; they provide mild acidification and are high in oxalate as well as vitamin C. 52
The diet should be adequately fortified with vitamin B6 because vitamin B6 deficiency promotes endogenous production and subsequent urinary excretion of oxalic acid. 53 There is no evidence that providing increased vitamin B6 beyond meeting the nutritional requirement provides a benefit in cats. Because most commercial diets designed for cats are well fortified with vitamin B6 it is unlikely that additional supplementation will be beneficial, except in homemade diets. Regardless, vitamin B6 is reasonably safe and sometimes provided to cats with persistent calcium oxalate crystalluria or frequent recurrences (see later).

Fiber
Increased intake of dietary fiber is associated with a decreased risk of calcium oxalate recurrence in some people, but not in cats unless they are hypercalcemic. Certain types of fiber (soy or rice bran) reduce calcium absorption from the gastrointestinal tract, which may decrease urinary calcium excretion. Also, higher fiber diets tend to be less acidifying. In five cats with idiopathic hypercalcemia and calcium oxalate uroliths, feeding a high fiber diet with supplemental potassium citrate resulted in normalization of serum calcium concentrations. 11 However, efficacy of increased fiber intake in cats is unproven at this time.
Preventive therapy – measures to increase urine volume
Increasing the urine volume is a mainstay of preventive therapy for calcium oxalate urolithiasis. By increasing water intake, urinary concentrations of calculogenic minerals are reduced. In addition, larger urine volumes typically hasten urine transit time and voiding frequency, thereby reducing retention time for crystal formation and growth.
Feeding a canned food containing at least 70% moisture54,55 is the most practical means of increasing water intake and lowering calcium oxalate urine saturation in cats. The goal is to dilute urine to a specific gravity of <1.040.7,46 Flavoring water, enhancing water access and adding water to dry foods are strategies that may be used in patients that refuse to eat canned foods. Sodium chloride may be used to increase water intake and several ‘calcium oxalate preventive’ diets contain >1% sodium chloride (DM basis).56,57 Increased dietary sodium may increase urinary calcium excretion, however, and can contribute to ongoing renal damage in cats with marginal renal function, 46 although this has not been a consistent finding.58,59
In cats, several diets are available that are formulated to reduce calcium and oxalic acid concentrations in urine, promote high concentration and activity of inhibitors of calcium oxalate crystal growth and aggregation in urine, and maintain dilute urine. Consumption of these diets by healthy cats results in production of urine that is undersaturated with calcium oxalate.50,60 In one study of cats with naturally occurring calcium oxalate uroliths, consumption of one such ‘preventive diet’ resulted in a decrease in urine saturation from the oversaturated state to a metastable state; uroliths did not recur in the cats. 44
In cats with hypercalcemia and calcium oxalate uroliths, prevention of recurrence appears to be more successful when feeding a higher fiber diet and administering potassium citrate (initial dose: 75 mg/kg PO q12h; adjust to induce a urine pH of 7.0–7.5). 11 Other treatments that have been proposed include vitamin B6 (2 mg/kg PO q24h) and hydrochlorothiazide (1–2 mg/kg PO q12h). A 65% decrease in urinary calcium and oxalate was reported in healthy cats receiving 1 mg/kg PO q12h of hydrochlorothiazide. 61
Key points
Urolithiasis occurs commonly in cats, with the majority of uroliths developing in the lower urinary tract.
More than 80–90% of lower urinary tract uroliths are calcium oxalate or struvite.
Calcium oxalate uroliths are not amenable to medical dissolution; therefore, removal by surgery or minimally invasive techniques must be performed, where required.
Preventive measures aim to decrease urinary calcium and oxalate excretion, increase urine volume, and induce a neutral to alkaline urinary pH.
Footnotes
Funding: The author received no financial support for the research, authorship and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Bartges JW, Osborne CA, Lulich JP, et al. Methods for evaluating treatment of uroliths. Vet Clin North Am Small Anim Pract 1999; 29: 45–57. [DOI] [PubMed] [Google Scholar]
- 2. Feeney DA, Anderson KL. Radiographic imaging in urinary tract disease. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames, IA: Wiley-Blackwell, 2011, pp 97–127. [Google Scholar]
- 3. Osborne CA, Lulich JP, Kruger JM, et al. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract 2009; 39: 183–197. [DOI] [PubMed] [Google Scholar]
- 4. Sturgess CP, Hesford A, Owen H, et al. An investigation into the effects of storage on the diagnosis of crystalluria in cats. J Feline Med Surg 2001; 3: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Langston C, Gisselman K, Palma D, et al. Diagnosis of urolithiasis. Compend Contin Educ Pract Vet 2008; 30: 447–450, 452–444; quiz 455. [PubMed] [Google Scholar]
- 6. Bartges JW. Urinary saturation testing. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames, IA: Wiley-Blackwell, 2011, pp 75–85. [Google Scholar]
- 7. Lulich JP, Osborne CA, Albasan H. Canine and feline urolithiasis: diagnosis, treatment, and prevention. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames, IA: Wiley-Blackwell, 2011, pp 687–706. [Google Scholar]
- 8. Seaman R, Bartges JW. Canine struvite urolithiasis. Compend Contin Educ Pract Vet 2001; 23: 407–429. [Google Scholar]
- 9. Palma D, Langston C, Gisselman K, et al. Canine struvite urolithiasis. Compend Contin Educ Pract Vet 2013; 35: E1; quiz E1. [PubMed] [Google Scholar]
- 10. Gisselman K, Langston C, Palma D, et al. Calcium oxalate urolithiasis. Compend Contin Educ Pract Vet 2009; 31: 496–502. [PubMed] [Google Scholar]
- 11. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc 1999; 35: 297–301. [DOI] [PubMed] [Google Scholar]
- 12. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000; 14: 619–626. [DOI] [PubMed] [Google Scholar]
- 13. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991–1997). J Vet Intern Med 2000; 14: 184–189. [DOI] [PubMed] [Google Scholar]
- 14. EH GC, Phillips H, Underwood L, et al. Risk factors for urolithiasis in dogs with congenital extrahepatic portosystemic shunts: 95 cases (1999–2013). J Am Vet Med Assoc 2015; 246: 530–536. [DOI] [PubMed] [Google Scholar]
- 15. Bartges JW, Cornelius LM, Osborne CA. Ammonium urate uroliths in dogs with portosystemic shunts. In: Bonagura JD. (ed). Current veterinary therapy XIII. Philadelphia: WB Saunders, 1999; 872–874. [Google Scholar]
- 16. Koehler LA, Osborne CA, Buettner MT, et al. Canine uroliths: frequently asked questions and their answers. Vet Clin North Am Small Anim Pract 2009; 39: 161–181. [DOI] [PubMed] [Google Scholar]
- 17. Kirk CA, Ling GV, Franti CE, et al. Evaluation of factors associated with development of calcium oxalate urolithiasis in cats. J Am Vet Med Assoc 1995; 207: 1429–1434. [PubMed] [Google Scholar]
- 18. Thumchai R, Lulich J, Osborne CA, et al. Epizootiologic evaluation of urolithiasis in cats: 3,498 cases (1982–1992). J Am Vet Med Assoc 1996; 208: 547–551. [PubMed] [Google Scholar]
- 19. Lekcharoensuk C, Lulich JP, Osborne CA, et al. Patient and environmental factors associated with calcium oxalate urolithiasis in dogs. J Am Vet Med Assoc 2000; 217: 515–519. [DOI] [PubMed] [Google Scholar]
- 20. Balaji KC, Menon M. Mechanism of stone formation. Urol Clin North Am 1997; 24: 1–11. [DOI] [PubMed] [Google Scholar]
- 21. Finch NC. Hypercalcaemia in cats. The complexities of calcium regulation and associated clinical challenges. J Feline Med Surg 2016; 18: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000; 14: 619–626. [DOI] [PubMed] [Google Scholar]
- 23. Bartges JW, Kirk C, Lane IF. Update: management of calcium oxalate uroliths in dogs and cats. Vet Clin North Am Small Anim Pract 2004; 34: 969–987, vii. [DOI] [PubMed] [Google Scholar]
- 24. McKerrell RE, Blakemore WF, Heath MF, et al. Primary hyperoxaluria (L-glyceric aciduria) in the cat: a newly recognised inherited disease. Vet Rec 1989; 125: 31–34. [DOI] [PubMed] [Google Scholar]
- 25. Gnanandarajah JS, Abrahante JE, Lulich JP, et al. Presence of Oxalobacter formigenes in the intestinal tract is associated with the absence of calcium oxalate urolith formation in dogs. Urol Res 2012; 40: 467–473. [DOI] [PubMed] [Google Scholar]
- 26. Dijcker JC, Kummeling A, Hagen-Plantinga EA, et al. Urinary oxalate and calcium excretion by dogs and cats diagnosed with calcium oxalate urolithiasis. Vet Rec 2012; 171: 646. DOI: 10.1136/vr.101130. [DOI] [PubMed] [Google Scholar]
- 27. Dijcker JC, Plantinga EA, van Baal J, et al. Influence of nutrition on feline calcium oxalate urolithiasis with emphasis on endogenous oxalate synthesis. Nutr Res Rev 2011: 24; 96–110. [DOI] [PubMed] [Google Scholar]
- 28. Ching SV, Fettman MJ, Hamar DW, et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J Nutr 1989; 119: 902–915. [DOI] [PubMed] [Google Scholar]
- 29. Sutton RA, Wong NL, Dirks JH. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 1979; 15: 520–533. [DOI] [PubMed] [Google Scholar]
- 30. Buffington CA, Rogers QR, Morris JG. Effect of diet on struvite activity product in feline urine. Am J Vet Res 1990; 51: 2025–2030. [PubMed] [Google Scholar]
- 31. Bartges JW, Kirk CA, Cox SK, et al. Influence of acidifying or alkalinizing diets on bone mineral density and urine relative supersaturation with calcium oxalate and struvite in healthy cats. Am J Vet Res 2013; 74: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 32. Bartges JW, Finco DR, Polzin DJ, et al. Pathophysiology of urethral obstruction. Vet Clin North Am Small Anim Pract 1996; 26: 255–264. [PubMed] [Google Scholar]
- 33. Lulich JP, Osborne CA, Sanderson SL, et al. Voiding urohydropropulsion: lessons from 5 years of experience. Vet Clin North Am Small Anim Pract 1999; 29: 283–292. [DOI] [PubMed] [Google Scholar]
- 34. McLoughlin MA. Complications of lower urinary tract surgery in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 889–913, v. [DOI] [PubMed] [Google Scholar]
- 35. Lulich JP, Osborne CA. Voiding urohydropropulsion. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames, IA: Wiley-Blackwell, 2011, pp 375–378. [Google Scholar]
- 36. Bartges JW, Sura PS, Callens A. Minilaparotomy-assisted cystotomy. In: Bonagura JD, Feldman EC. (eds). Current veterinary therapy XV. St Louis, MO: Saunders-Elsevier, 2014, pp 905–909. [Google Scholar]
- 37. Rawlings CA. Diagnostic rigid endoscopy: otoscopy, rhinoscopy, and cystoscopy. Vet Clin North Am Small Anim Pract 2009; 39: 849–868. [DOI] [PubMed] [Google Scholar]
- 38. Lulich JP, Osborne CA, Albasan H, et al. Efficacy and safety of laser lithotripsy in fragmentation of urocystoliths and urethroliths for removal in dogs. J Am Vet Med Assoc 2009; 234: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 39. Lulich JP, Adams LG, Grant D, et al. Changing paradigms in the treatment of uroliths by lithotripsy. Vet Clin North Am Small Anim Pract 2009; 39: 143–160. [DOI] [PubMed] [Google Scholar]
- 40. Bevan JM, Lulich JP, Albasan H, et al. Comparison of laser lithotripsy and cystotomy for the management of dogs with urolithiasis. J Am Vet Med Assoc 2009; 234: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 41. Grant DC, Werre SR, Gevedon ML. Holmium:YAG laser lithotripsy for urolithiasis in dogs. J Vet Intern Med 2008; 22: 534–539. [DOI] [PubMed] [Google Scholar]
- 42. Adams LG, Berent AC, Moore GE, et al. Use of laser lithotripsy for fragmentation of uroliths in dogs: 73 cases (2005–2006). J Am Vet Med Assoc 2008; 232: 1680–1687. [DOI] [PubMed] [Google Scholar]
- 43. Davidson EB, Ritchey JW, Higbee RD, et al. Laser lithotripsy for treatment of canine uroliths. Vet Surg 2004; 33: 56–61. [DOI] [PubMed] [Google Scholar]
- 44. Lulich JP, Osborne CA, Lekcharoensuk C, et al. Effects of diet on urine composition of cats with calcium oxalate urolithiasis. J Am Anim Hosp Assoc 2004; 40: 185–191. [DOI] [PubMed] [Google Scholar]
- 45. Lekcharoensuk C, Lulich JP, Osborne CA, et al. Association between patient-related factors and risk of calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc 2000; 217: 520–525. [DOI] [PubMed] [Google Scholar]
- 46. Kirk CA, Ling GV, Osborne CA, et al. Clinical guidelines for managing calcium oxalate uroliths in cats: medical therapy, hydration, and dietary therapy. Managing urolithiasis in cats: recent updates and practice guidelines. Topeka, KS: Hill’s Pet Nutrition, 2003; pp 10–19. [Google Scholar]
- 47. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Association between dietary factors and calcium oxalate and magnesium ammonium phosphate urolithiasis in cats. J Am Vet Med Assoc 2001; 219: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 48. Robertson WG. Urinary calculi. In: Nordin BEC, Need AG, Morris HA. (eds). Metabolic bone and stone disease. Edinburgh: Churchill Livingstone, 1993, pp 249–311. [Google Scholar]
- 49. Lulich JP, Osborne CA, Bartges JW, et al. Canine lower urinary tract disorders. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 5th ed. Philadelphia: WB Saunders, 1999, pp 1747–1783. [Google Scholar]
- 50. Smith BHE, Moodie SJ, Wensley S, et al. Differences in urinary pH and relative supersaturation values between senior and young adult cats [abstract]. J Vet Intern Med 1997; 11: 127. [Google Scholar]
- 51. Passlack N, Burmeier H, Brenten T, et al. Relevance of dietary protein concentration and quality as risk factors for the formation of calcium oxalate stones in cats. J Nutr Sci 2014; 3: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Terris MK, Issa MM, Tacker JR. Dietary supplementation with cranberry concentrate tablets may increase the risk of nephrolithiasis. Urology 2001; 57: 26–29. [DOI] [PubMed] [Google Scholar]
- 53. Bai SC, Sampson DA, Morris JG, et al. Vitamin B6 requirement of growing kittens. J Nutr 1989; 119: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 54. Buckley CM, Hawthorne A, Colyer A, et al. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br J Nutr 2011; 106 Suppl 1: S128–130. [DOI] [PubMed] [Google Scholar]
- 55. Deng P, Iwazaki E, Suchy SA, et al. Effects of feeding frequency and dietary water content on voluntary physical activity in healthy adult cats. J Anim Sci 2014; 92: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 56. Lulich JP, Osborne CA, Sanderson SL. Effects of dietary supplementation with sodium chloride on urinary relative supersaturation with calcium oxalate in healthy dogs. Am J Vet Res 2005; 66: 319–324. [DOI] [PubMed] [Google Scholar]
- 57. Stevenson AE, Hynds WK, Markwell PJ. Effect of dietary moisture and sodium content on urine composition and calcium oxalate relative supersaturation in healthy miniature schnauzers and labrador retrievers. Res Vet Sci 2003; 74: 145–151. [DOI] [PubMed] [Google Scholar]
- 58. Xu H, Laflamme DP, Long GL. Effects of dietary sodium chloride on health parameters in mature cats. J Feline Med Surg 2009; 11: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chetboul V, Reynolds BS, Trehiou-Sechi E, et al. Cardiovascular effects of dietary salt intake in aged healthy cats: a 2-year prospective randomized, blinded, and controlled study. PLoS One 2014; 9: e97862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith BH, Stevenson AE, Markwell PJ. Urinary relative supersaturations of calcium oxalate and struvite in cats are influenced by diet. J Nutr 1998; 128: 2763S-2764S. [DOI] [PubMed] [Google Scholar]
- 61. Hezel A, Bartges JW, Kirk CA, et al. Influence of hydrochlorothiazide on urinary calcium oxalate relative supersaturation in healthy young adult female domestic shorthaired cats. Vet Ther 2007; 8:247–254. [PubMed] [Google Scholar]