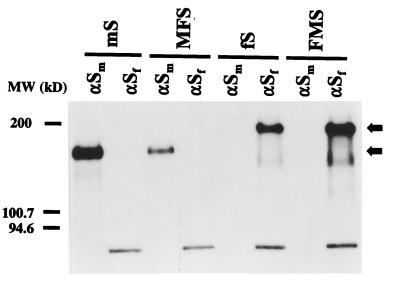
FIG. 2.
Expression of chimeric spike proteins. Parallel cultures of OST7-1 cells in 35-mm-diameter dishes were infected with vTF7-3 and transfected with plasmids encoding the wild-type and chimeric S proteins described in the legend to Fig. 1. Cells were incubated at 32°C. Starting at 4.5 h p.i., they were starved for 30 min in cysteine- and methionine-free minimal essential medium containing 10 mM HEPES (pH 7.2) without fetal bovine serum. The medium was then replaced by 600 μl of the same containing 100 μCi of 35S in vitro cell labeling mix (Amersham). After a 1-h labeling period, cells were washed with phosphate-buffered saline and solubilized in 1 ml of lysis buffer, TES (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA) containing 1% Triton X-100 and 2 mM phenylmethylsulfonyl fluoride. Nuclei were removed from the cell lysates by centrifugation at 12,000 × g for 10 min at 4°C. For immunoprecipitations, 50-μl aliquots of lysate were diluted with 1 ml of detergent solution (50 mM Tris-HCl [pH 8.0], 62.5 mM EDTA, 0.5% Nonidet P-40, 0.5% Na deoxycholate), and 30 μl of 10% sodium dodecyl sulfate was added. MAbs were then added: 3 μl of hybridoma culture supernatant WA3.10 or 23F4.5, which recognizes the S protein of MHV (αSm) or FIPV (αSf), respectively. Following an overnight incubation at 4°C, immune complexes were adsorbed for 1 h to formalin-fixed Staphylococcus aureus cells (BRL Life Technologies) added as 45 μl of a 10% (wt/vol) suspension. Immune complexes were collected by centrifugation at 12,000 × g and washed three times with radioimmunoprecipitation assay buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and 1% Na deoxycholate). Pellets were resuspended in 30 μl of Laemmli sample buffer, heated for 2.5 min at 95°C, and analyzed by electrophoresis in a sodium dodecyl sulfate–12.5% polyacrylamide gel followed by fluorography. MW, molecular mass.