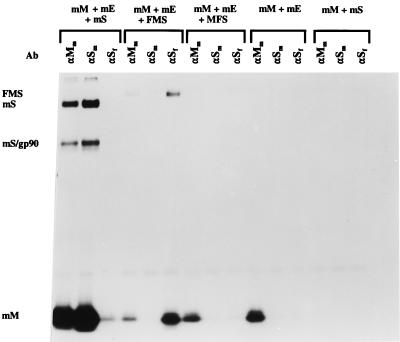
FIG. 4.
Incorporation of chimeric FMS into MHV-based VLPs. Parallel cultures of OST7-1 cells in 35-mm-diameter dishes were infected with vTF7-3 and transfected with different combinations of plasmids as indicated (mS, mE, and mM represent plasmids encoding the wild-type MHV-A59 S, E, and M proteins, respectively; FMS and MFS refer to plasmids encoding the chimeric S proteins described in the legend to Fig. 1). Cells were incubated at 32°C and labeled from 5 to 8 h p.i. with 35S-amino acids (100 μCi/dish). Culture media (0.8 ml) were harvested, cleared by low-speed centrifugation, mixed with 2.3 ml of 67% sucrose in TM (10 mM Tris-HCl [pH 7.0], 10 mM MgCl2), and transferred into Beckman SW50.1 ultracentrifuge tubes. Each solution was overlaid with 1 ml of 48% sucrose, 0.5 ml of 40% sucrose, and 0.5 ml of 30% sucrose in TM, and the gradients were centrifuged at 36,000 rpm for 43 h. After centrifugation, a fraction consisting of the top 1 ml of each tube was collected. Virus particles were affinity purified from 150 μl of this fraction by addition of 25 μl of MAb J1.3 against the MHV M protein (αMm); 10 μl of MAb WA3.10, which is directed against an epitope in the MHV S ectodomain (αSm); or 3 μl of MAb 23F4.5, which recognizes an epitope in the FIPV S ectodomain (αSf). Samples were processed and analyzed as described for Fig. 2 except that the Staphylococcus aureus immune complexes were washed once with TM instead of three times with radioimmunoprecipitation assay buffer. At the left of the figure, mS and mS/gp90 indicate the positions of the uncleaved and cleaved forms of the S protein, respectively; mM and FMS mark the positions of the M protein and the chimeric S protein, respectively. Ab, antibody.