Abstract
Retinoids are used topically as well as orally, and the most commonly used oral retinoids are isotretinoin and acitretin. Mucocutaneous adverse effects are frequently seen with the use of systemic retinoids, the most common being cheilitis, which is dose-dependent and seen in almost all patients using it. To study the comparative effect of topical tacrolimus 0.1% ointment versus topical white soft petrolatum jelly in the treatment of cheilitis due to retinoids. A total of 26 patients with cheilitis post-treatment with isotretinoin were enrolled in this cross-sectional study conducted over a period of 6 months. They were randomized into two groups of 13 patients each to receive topical tacrolimus 0.1% ointment and soft petrolatum jelly twice daily, respectively. Patients were followed up weekly with clinical photographs. Resolution of cheilitis was assessed on the basis of photograph and ICGS score. About 84.6% of patients of group A and 53.8% of patients of group B showed resolution of symptoms within 1 week of treatment. A significant difference was seen in duration for complete cheilitis resolution and relapse rate in the two groups. Our study concludes that oral retinoid-induced cheilitis shows faster and more significant resolution with twice-daily topical tacrolimus 0.1% ointment application compared to twice-daily topical petrolatum jelly.
Keywords: ICGS, isotretinoin, topical tacrolimus, petrolatum jelly
Introduction
The use of retinoids orally as well as topically is rampant in dermatology; the two most commonly used oral ones are isotretinoin (first generation) and acitretin (second generation). Vitamin A is absorbed, stored and metabolized by the skin. The differentiation and cell division of the epidermis’ stratified structures are influenced by retinoids. Retinoids have a major impact on a number of skin physiological responses, including wound healing, immunological response and dermal ageing.[1]
One significant group of adverse effects of oral retinoid therapy is mucocutaneous toxicity, which prevents further dose escalation. The most frequent and annoying mucocutaneous side effect of oral retinoid therapy is cheilitis (lip chapping). Understanding the fundamental pathogenesis of retinoid-induced cheilitis is essential for rational and practical management of the condition.[2]
Lip inflammation known as cheilitis can be either acute or persistent. The vermilion zone is where the inflammation first appears, though it can also affect nearby skin and, less frequently, the oral mucosa. Numerous causes, such as contact irritants or allergens, prolonged sun exposure, dietary inadequacies, as well as other cutaneous and systemic disorders, may contribute to its development.
Nearly, all patients who use systemic retinoids, such as etretinate and isotretinoin, develop dryness, erythema, scaling and cracking of the lips (which may extend to the angles of the mouth). Less frequently, any other medications that produce dry mouth and lips could be the culprit.[3]
Various treatment modalities tried for cheilitis include lip moisturizers, sunscreens, topical and systemic vitamin E, topical tacrolimus and topical low-potency corticosteroids, either alone or in combination.[2]
Hence, this comparative study of topical tacrolimus 0.1% ointment and soft petrolatum jelly was conducted in oral retinoid-induced cheilitis patients.
Material and Methods
After obtaining the Clinical Trials Registry – India (CTRI) approval (CTRI/2022/01/039574), a written informed consent and Institutional Ethics Committee (IEC) clearance, 26 patients on 0.75 mg–1 mg/kg oral isotretinoin with isotretinoin-induced cheilitis after the use were enrolled in this cross-sectional type of study for a duration of 6 months. The onset of cheilitis was noted approximately at 2 weeks.
Patients with a past history of cheilitis, pregnant or lactating females, under the age of 18 and those having immunosuppression due to any disease/drug were excluded from the study. They were divided equally into two groups on the basis of computer-generated randomization. Group A patients (n = 13) applied topical tacrolimus 0.1% ointment twice daily, while group B patients (n = 13) applied white soft petrolatum jelly twice daily.
Patients were followed up weekly with clinical photographs. The parameters taken into consideration for assessment were as follows: i) time taken for the development of cheilitis after treatment with oral retinoids; ii) grade of cheilitis; iii) time required for resolution of cheilitis in patients using topical tacrolimus 0.1% ointment (group A); and iv) time required for resolution of cheilitis in patients using white soft petrolatum jelly (group B). Resolution of cheilitis was assessed on the basis of photograph and ICGS incorporating the following four characteristics: erythema, scale/crust, fissures and inflammation of commissures, each ranked 0–3. The total score was calculated and cheilitis was graded as mild (ICGS score, 0–4), moderate (ICGS score, 5–8) and severe (ICGS score, 9–12).
Patients were sorted by simple randomization into two groups, and participant blinding was conducted.
Allocation concealment was conducted by central randomization.
Results
The mean age of patients who participated in our study was 26.08 ± 4.33 years, and the mean duration required for the development of cheilitis after starting isotretinoin was 6.77 ± 3.01 days.
Eleven (84.6%) participants of group A showed resolution of symptoms within 1 week of treatment, with resolution seen earliest after two applications or 1 day. Seven (53.8%) participants of group B showed resolution of symptoms within 1 week, with resolution seen earliest after six applications or 3 days. Two participants of this group failed to show complete resolution even after 4 weeks.
In a patient who developed cheilitis grade 2, pre-treatment score 6, 3 days after treatment with oral isotretinoin, complete resolution of cheilitis, that is grade 1, was seen after two applications of tacrolimus 0.1% ointment [Figure 1a and b], whereas in a patient who developed cheilitis grade 2, pre-treatment score 8, 3 days after treatment with oral isotretinoin, no resolution of cheilitis was seen after twice-daily application of white soft petrolatum jelly for 4 weeks [Figure 2a and b]. There was a statistically significant difference in the post-treatment ICGS score and duration for complete resolution of cheilitis in the two groups [Table 1]. However, the therapy was continued on SOS basis if symptoms reoccurred till the patients were on isotretinoin therapy.
Figure 1.
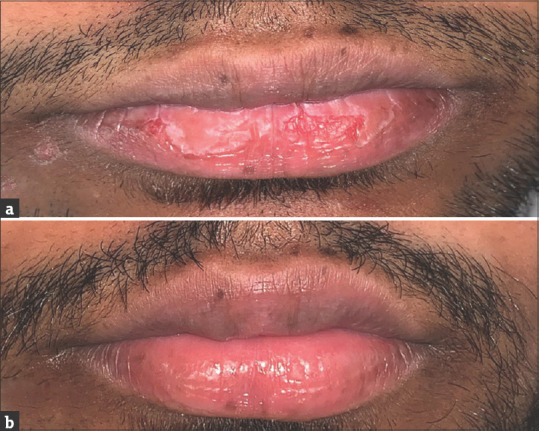
(a) A 21-year-old male patient who developed cheilitis of grade 2 pre-treatment score 6 (E:2, S/C:2 F:1 C:1), 3 days after treatment with oral isotretinoin. (b) There was complete resolution of cheilitis, that is grade 1 and post-treatment score 0 (E:0, S/C:0 F:0 C:0), after two applications of tacrolimus 0.1% ointment
Figure 2.
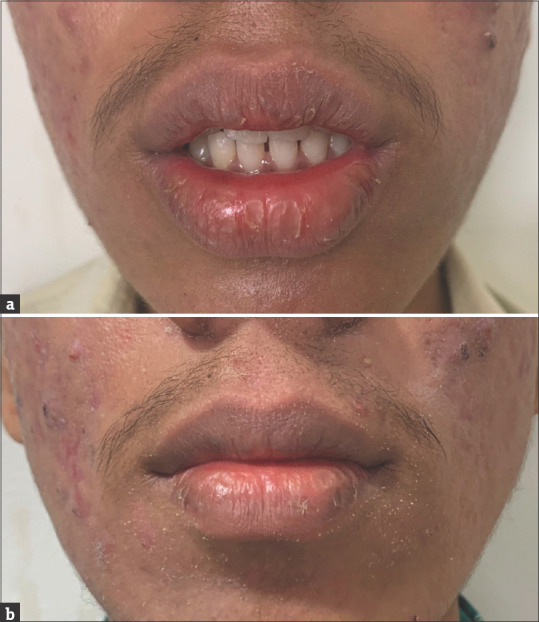
(a) A 26-year-old male patient who developed cheilitis grade 2 pre-treatment. (b) A 26-year-old male patient who developed cheilitis grade 2 post-treatment
Table 1.
Comparison of age, pre- and post-treatment ICGS score, duration for the development of cheilitis and complete resolution in the two groups
| Group A (n=13) | Group B (n=13) | P | |
|---|---|---|---|
| Age (years) | 26.62±4.48 | 25.54±4.27 | 0.5366 |
| Duration for development of cheilitis (days) | 6.54±3.07 | 7±3.06 | 0.7043 |
| Pre-treatment ICGS score | 7±1.58 | 7.08±1.75 | 0.9075 |
| Post-treatment ICGS score | 1.92±1.32 | 2.54±2.37 | 0.6585 |
| Duration for complete resolution (days) | 4.38±2.72 | 10.27±4.41 | 0.0006 |
| Relapse | 5 (38.46%) | 8 (72.7%) | 0.047 |
Discussion
Our study was conducted for a duration of 6 months and included 26 patients who have developed cheilitis post-treatment with oral isotretinoin. Resolution of cheilitis was assessed on the basis of various parameters and ICGS scoring scale. Patients in group A who were administered topical tacrolimus 0.1% ointment twice daily showed resolution of symptoms within 1 week of treatment with the earliest resolution seen after two applications or 1 day.
In the case of patients of group B who were administered to use white soft petrolatum jelly twice daily, they showed resolution of symptoms within 1 week with the earliest seen after six applications or 3 days.
Connolly M and Kennedy C reported a case of a recalcitrant exfoliative cheilitis of 8-month duration, which was non-responsive to treatment with topical corticosteroids and moisturizers. It responded to treatment with topical tacrolimus.[4]
A study conducted by Qian-Qian Zhang concluded that the application of topical tacrolimus 0.1% ointment once a day had similar clinical improvement compared to once-in-2-day application in the treatment of cheilitis.[5]
Our study concluded that oral retinoid-induced cheilitis shows faster and more significant resolution with twice-daily topical tacrolimus 0.1% ointment application compared to twice-daily application of topical soft white petrolatum jelly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sarkar R, Chugh S, Garg VK. Acitretin in dermatology. Indian J Dermatol Venereol Leprol. 2013;79:759–71. doi: 10.4103/0378-6323.120721. [DOI] [PubMed] [Google Scholar]
- 2.Madke B, Shah H, Singh AL Khoja M, Kabra P. Oral retinoid-induced cheilitis. Indian J Drugs Dermatol. 2016;2:50–3. [Google Scholar]
- 3.Bhutta BS, Hafsi W. Stat Pearls. Treasure Island (FL): Stat Pearls Publishing; 2020. [[Updated 2020 Apr 24]]. Cheilitis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470592/ [Google Scholar]
- 4.Connolly M, Kennedy C. Exfoliative cheilitis successfully treated with topical tacrolimus. Br J Dermatol. 2004;151:241–2. doi: 10.1111/j.1365-2133.2004.06043.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q-Q, Xu P, Sun C, Liu LJ, Jiang WW. Topical tacrolimus with different frequency for exfoliative cheilitis: A pilot study. J Dermatol Treat. 2022;33:550–4. doi: 10.1080/09546634.2020.1771258. [DOI] [PubMed] [Google Scholar]


