Abstract
Background:
Limited data exist on the circadian blood pressure (BP) and heart rate (HR) variations that occur in heart failure (HF) patients on left ventricular assist device (LVAD) support.
Methods:
We prospectively recorded clinic and 24-hour ambulatory BP and HR data in patients on HeartMate II LVAD support. Results were compared to HF patients with ejection fraction ≤30% and controls with no history of cardiovascular disease. Physiologic nocturnal BP and HR dipping was defined as a ≥10 decline compared to daytime values.
Result:
Twenty-nine LVAD patients (age 59±15y, 76% male, 38% ischemic etiology), 25 HF patients (age 64±13y, 84% male, 32% ischemic etiology) and 26 controls (age 56±9y, 62% male) were studied. Normal nocturnal BP dipping was less frequent in LVAD patients (10%) than in HF patients (28%) and controls (62%) and reversed BP dipping (BP increase at night) was more common in LVAD patients (24%), compared to HF (16%) and controls (8%), (p<0.001, for all comparisons). Physiologic HR reduction was less frequent in LVAD patients (14%), compared to HF (16%) and controls (59%) (p<0.001, for all comparisons). Among LVAD patients, 36% exhibited sustained hypertension over the 24-hours and 25% had white-coat hypertension.
Conclusions:
Treatment of advanced HF with an LVAD does not restore physiologic circadian variability of BP and HR; additionally, BP was not adequately controlled in more than a third of LVAD patients, and a quarter of them exhibited white-coat hypertension. Future studies are warranted to confirm these findings and investigate prognostic and management implications in this population.
Keywords: ABPM, LVAD, 24-hour blood pressure, ventricle assist device, circadian variability, night dipping
Introduction
Circadian blood pressure (BP) and heart rate (HR) variability represents an evolutionary mechanism (1) controlled by the interactions of different environmental, neural and neurohumoral components (2). In healthy subjects, a nocturnal reduction (dipping) of BP and HR (3) modulates the cardiac metabolism throughout the 24 hours (4). In heart failure (HF) patients, this nocturnal dipping in BP and HR is frequently reduced or lost, resulting in abnormal circadian patterns that are associated with worse clinical outcomes (5–7). The pathogenesis of these abnormal BP and HR profiles has been linked to the sympathetic and neurohormonal activation, vascular dysfunction and impaired baroreflex activity that characterize the HF syndrome (8, 9).
In patients with end-stage HF, left ventricular assist devices (LVADs) have progressively become an alternative to transplant, either as destination therapy or bridge to transplantation (10). Although LVADs have been showed to improve cardiac output and peripheral perfusion (10), limited data exist on the ability of LVAD therapy to restore normal BP and HR circadian variability among HF patients. In addition, 24-hour BP monitoring can identify BP profiles, such as white-coat hypertension (HTN) (elevated BP in clinic, but normal BP over the 24 hours) and masked HTN (normal BP in clinic, but elevated BP over the 24 hours) that have been used to guide medical management in patients with cardiovascular disease(11). The frequency of these HTN phenotypes has never been described in the LVAD population.
Here, we studied the 24-hour ambulatory BP and HR profiles of LVAD patients, and compared them to those of HF patients with severely reduced left ventricular ejection fraction (LVEF) and a control group of subjects with no known history of cardiovascular disease.
Methods
Study Design and Participants
In this cohort study, we prospectively enrolled 26 control subjects with no documented or reported history of cardiovascular disease (Controls), 25 patients with HF and severely reduced LVEF, and 29 patients implanted with an LVAD between September 2015 and May 2019.
Control subjects were studied at Cardiff Metropolitan University (UK), where participants >18 years old were screened and recruited as part of a free cardiovascular health awareness and screening program offered to members of the public. Clinical data, past medical history, and pharmacological history were reviewed by the investigators to confirm that all control subjects included were free from cardiovascular acting medications and history of cardiovascular diseases. Ambulatory HF patients with an LVEF ≤30% and LVAD patients on HeartMate II (HMII; Abbott, Abbott Park, Illinois, USA) were identified and enrolled in the HF clinic at Columbia University Irving Medical Center (USA). Clinical data were extrapolated from the electronic medical records. The study protocol was approved by the Cardiff Metropolitan University ethics committee and the Columbia University Institutional Review Board. The study was conducted in compliance with the ISHLT ethics statement.
Clinic BP and HR measurements
For healthy controls, clinic BP and HR measurements were performed at the time of enrolment into the cardiovascular health awareness and screening program. For HF and LVAD patients, clinic BP and HR measurements were performed during a routine outpatient visit. We utilized the Mobil-O-Graph (IEM, Germany), an ambulatory blood pressure monitor (ABPM), validated in both non LVAD(12, 13) and HMII LVAD patients (14), for all the study participants. An appropriately sized BP cuff was placed around the non-dominant arm at the level of the heart. Measurements were obtained in a quiet environment. Before each measurement, participants sat comfortably for at least five minutes with the arm and back supported, as recommended by guidelines (15). Two measurements were obtained in each participant at 5-minute intervals; the last measurement was used for analysis of clinic BP and HR.
ABPM measurements
Measurements were obtained using the Mobil-O-Graph device according to current guidelines (11). After the device was configured and positioned during the clinic visit, participants returned to their usual daily activities. Participants were instructed to keep their arm still and relaxed during the measurements. Systolic BP (SBP), diastolic BP (DBP), mean arterial BP (MAP), pulse pressure (PP) and HR were automatically recorded. Measurements were set every 30 minutes during the day-time and every 60-minutes during the night-time in all subjects. Day- and night-time periods were individually tailored for each participant at beginning of the recording, based on reported usual sleep time. The ABPM device was returned or collected at the patient’s home following the 24-hour study period.
In a subgroup of patients (n=23, 29%), we also obtained measurements every 15 minutes to test whether higher sampling rate could improve accuracy of 24-hour day-time and night-time BP assessment (11). In these patients, 24-hour, day- and night-time SBP, DBP, and HR readings obtained every 15 minutes were compared to readings taken every 30- and 60-minute by filtering the same BP log for these standard diurnal and nocturnal intervals. Average measurements obtained at 15-minute strongly correlated with those obtained at 30- and 60-minute intervals as shown in the Supplemental materials.
Blood pressure and HR night dipping
Subjects who had a nocturnal decline of SBP ≥10% were defined as SBP dippers (11). Similarly, subjects who had a nocturnal decline of DBP ≥10% were defined as DBP dippers (11). Participants with any increase of BP (SBP or DBP) during the night period were considered as reverse dippers (11). Based on circadian HR changes, patients were similarly classified as HR dippers or reverse dippers.
Hypertensive thresholds
In controls and HF patients, HTN was defined as an average BP ≥130/80 mmHg during the 24-hour period, and/or ≥135/85 during the day, and/or ≥120/70 during the night (11). For LVAD patients where ABPM threshold for HTN is not clearly defined, we arbitrarily chose a cut off of MAP >90 mmHg over the 24-hour, since this value in LVAD patients during clinic assessment has been previously associated with the thromboembolic complications in outpatients on LVAD support (16, 17).
Hypertension phenotypes
Based on clinic and ambulatory BP measurements, four different BP phenotypes were identified: sustained HTN [elevated BP during the clinic visit (BP ≥140/90 mmHg in non-LVAD patients, MAP >90 mmHg in LVAD patients) and elevated 24-hour BP, as defined above](11); masked HTN [normal BP during the visit (BP <140/90 mmHg in non-LVAD patients, MAP ≤90 mmHg in LVAD patients) and elevated BP during the 24-hour period, the day- or the night-time]; 3) white-coat HTN [elevated BP during the visit and normal BP during all the 24-hour time periods]; and 4) sustained normotension [normal clinic and ambulatory BP levels] (11).
Statistical methods
All analyses were performed in SPSS (Armonk, NY: IBM Corp) version 25. Descriptive data are presented as proportions or means ± SD (unless otherwise specified). The normality of data was assessed using the Shapiro-Wilk test. Differences among the groups were assessed using the one-way ANOVA test for normally distributed variables and Kruskal Wallis test for non-normally distributed variables. Pearson’s χ2 test or Fisher’s exact test were used to compare categorical variables, as appropriate. For post-hoc analysis, Bonferroni-corrected Student’s t-test or Bonferroni-corrected Mann Whitney U-test were used, based on normality. Difference between day and night values was assessed with paired t-test or Wilcoxon signed-rank test, as appropriate. A value of p<0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline demographic and clinical characteristics are presented in Table 1. Participants had similar age, sex distribution and body mass index. Among HF patients, average left ventricular end diastolic diameter and LVEF were 6.5±1.0 cm and 18.2±5.7%, respectively. Among LVAD patients, average speed, flow, PI, and power were 9091±488 rpm, 4.9±0.9 l/min, 6.2±1.3, and 5.4±1.1, respectively. Average duration on LVAD support was 749±753 days.
Table 1.
Baseline clinical characteristics
| Controls | HF | LVAD | |
|---|---|---|---|
| n=26 | n=25 | n=29 | |
| Demographics | |||
| Age, years | 55.6±9.4 | 64.2±12.6 | 59.0±15.3 |
| Male, n (%) | 16/26 (62) | 21/25 (84) | 22/29 (76) |
| BMI, m/kg2 | 27.2±4.4 | 27.0±4.8 | 29.0±7.3 |
| Ischemic etiology, n (%) | -- | 8/25 (32) | 11/29 (38) |
| LVEDD, cm | -- | 6.5±1.0 | 5.6±1.1 |
| LVEF, % | -- | 18.2±5.7 | 21.1±12.2 |
| BTT, n (%) | -- | -- | 9/29 (31) |
| Speed, rpm | -- | -- | 9091±488 |
| Flow, l/min | -- | -- | 4.9±0.9 |
| PI | -- | -- | 6.2±1.3 |
| Power, W | -- | -- | 5.4±1.1 |
| Time on LVAD support, days | -- | -- | 749±753 |
| Time after OHT, days | -- | -- | -- |
| Past Medical History | |||
| Hypertension, n (%) | -- | 16/25 (64) | 16/29 (55) |
| Diabetes, n (%) | -- | 6/25 (24) | 15/29 (52) |
| Chronic kidney diseases, n (%) | -- | 4/25 (16) | 5/29 (17) |
| Dyslipidemia, n (%) | -- | 10/25 (40) | 10/29 (35) |
| Atrial fibrillation, n (%) | -- | 8/25 (32) | 7/29 (24) |
| Asthma/COPD, n (%) | -- | 4/25 (16) | 8/29 (28) |
| Venous thromboembolism, n (%) | -- | 2/25 (8) | 3/29 (10) |
| Cardiovascular accident, n (%) | -- | 3/25 (12) | 1/29 (3) |
| Peripheral vascular disease, n (%) | -- | 0/25 (0) | 2/29 (7) |
| Hypothyroidism, n (%) | -- | 3/25 (12) | 6/29 (21) |
| Malignancy, n (%) | -- | 6/25 (24) | 8/29 (28) |
| Medications | |||
| ACE-I, n (%) | -- | 7/25 (28) | 7/29 (21) |
| ARB, n (%) | -- | 2/25 (8) | 5/29 (17) |
| Sacubitril / Valsartan, n (%) | -- | 13/25 (52) | 1/29 (3) |
| Calcium channel blocker, n (%) | -- | 1/25 (4) | 7/29 (24) |
| Aldosterone receptor antagonist, n (%) | -- | 14/25 (56) | 13/29 (48) |
| Beta blocker, n (%) | -- | 25/25 (100) | 26/29 (90) |
| Digoxin, n (%) | -- | 10/25 (40) | 7/29 (24) |
| Diuretic, n (%) | -- | 15/25 (60) | 18/29 (62) |
Mean ± standard deviation. ACE-I = Angiotensin-converting enzyme inhibitor; ARB= Angiotensin II receptor blocker; BMI = Body Mass Index; BTT = Bridge to Transplant; COPD = Chronic obstructive pulmonary disease; HF = Heart Failure; HTN = Hypertension; LVAD = Left Ventricle Assist Device; LVEDD = Left Ventricle End Diastolic Diameter; LVEF = Left Ventricle Ejection Fraction; PI = Pulsatility Index.
BP and HR values
Blood pressure and HR values at the clinic visit and over the 24 hours are presented and compared in Figure 1A and 1B, respectively, and in Supplemental Table 1. LVAD patients had the lowest SBP in clinic and the lowest PP, both in clinic and over the 24-hour period. DBP in clinic did not significantly differ among groups, while it was significantly lower in the HF group compared to the other two groups over the 24-hour period. Controls had the highest MAP over the 24-hour period.
Figure 1. A) Blood pressure and heart rate values at the clinic visit. B) Average blood pressure and heart rate values over the 24 hours.
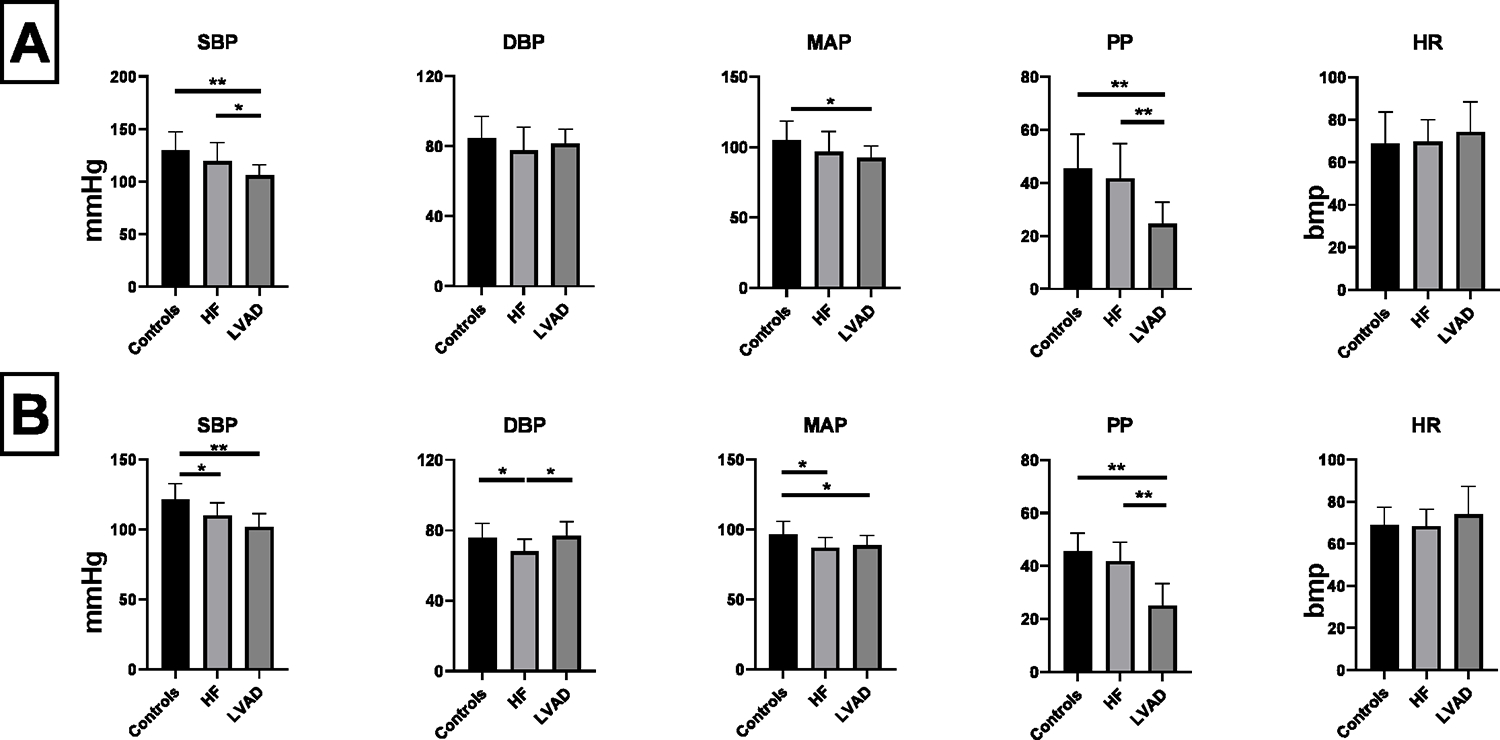
Bpm = Beats per Minute; DBP = Diastolic Blood Pressure; HF = Heart Failure;; HR = Heart Rate; LVAD = Left Ventricle Assist Device; MAP = Mean Arterial Pressure; PP = Pulse Pressure; SBP = Systolic Blood Pressure. * = p<0.05. ** = p<0.01. Comparisons between groups performed with Bonferroni-corrected Student’s t-test or Bonferroni-corrected Mann–Whitney U-test.
Circadian (day vs night) BP and HR variation and dipping profiles
Circadian data are presented in Table 2 and Figure 2. Control subjects had the greatest absolute reduction in SBP, DBP, MAP, PP and HR at night. HF and LVAD patients showed a statistically significant decline in SBP, DBP, MAP, but not in PP. Nocturnal HR was significantly lower than day-time HR in all groups except in LVAD patients. Relative changes in BP and HR are presented in Supplemental Figure 1. Absolute changes in BP and HR, and between-group comparisons are shown in Supplemental Table 2 and Supplemental Table 3, respectively. In brief, the percent reduction in SBP and DBP was less prominent in HF and LVAD patients compared to controls. When compared to HF, the LVAD group had a similar reduction in SBP, but less reduction in DBP.
Table 2.
Circadian changes in blood pressure and heart rate values.
| Controls | HF | LVAD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | Night | p-value | Day | Night | p-value | Day | Night | p-value | |
| SBP, mmHg | 126±12 | 111±9 | <0.001 | 112±10 | 106±11 | 0.005 | 104±9 | 98±10 | <0.001 |
| DBP, mmHg | 80±9 | 68±8 | <0.001 | 71±7 | 63±8 | <0.001 | 78±8 | 74±9 | <0.001 |
| MAP, mmHg | 101±10 | 88±7 | <0.001 | 90±7 | 83±9 | <0.001 | 90±7 | 85±9 | <0.001 |
| PP, mmHg | 46±9 | 43±8 | 0.01 | 42±8 | 43±7 | 0.068 | 25±8 | 24±9 | 0.069 |
| HR, bpm | 72±9 | 65±10 | <0.001 | 70±9 | 67±9 | 0.001 | 74±14 | 74±14 | 0.293 |
Mean ± standard deviation. Bpm = Beats per Minute; DBP = Diastolic Blood Pressure; HF = Heart Failure; HR = Heart Rate; LVAD = Left Ventricle Assist Device; MAP = Mean Arterial Pressure; PP = Pulse Pressure; SBP = Systolic Blood Pressure. Comparisons of Day vs Night values performed with paired t-test or Wilcoxon signed-rank test.
Figure 2. Circadian variation of blood pressure and heart rate values.
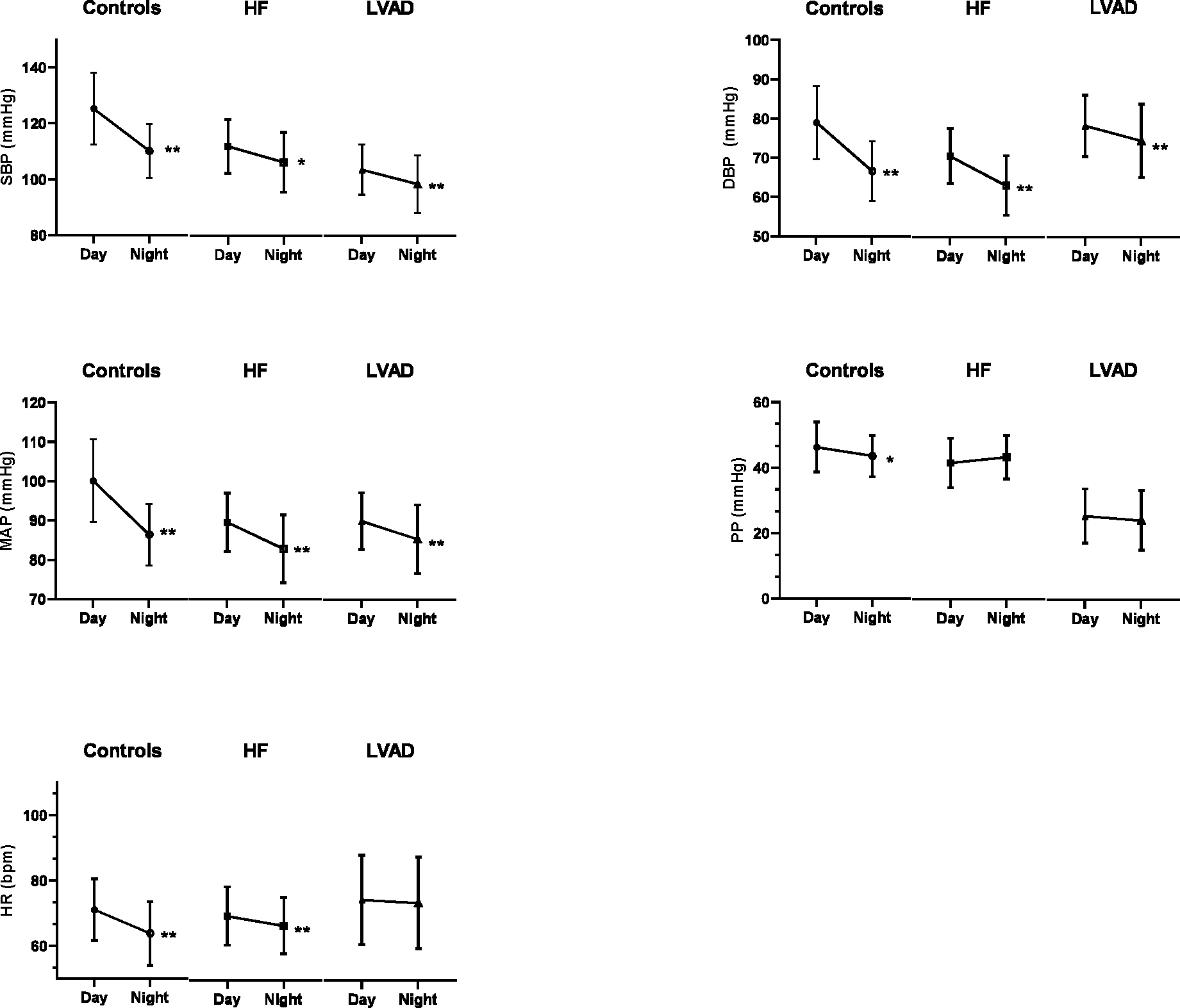
Bpm = Beats per Minute; DBP = Diastolic Blood Pressure; HF = Heart Failure; HR = Heart Rate; LVAD = Left Ventricle Assist Device; MAP = Mean Arterial Pressure; PP = Pulse Pressure; SBP = Systolic Blood Pressure. * = p<0.05. ** = p<0.001. Comparisons of Day vs Night values performed with paired t-test or Wilcoxon signed-rank test
The percentages of subjects experiencing SBP and DBP dipping, as well as a reverse dipping phenomena, are presented in Table 3A. Distribution of BP dipping profiles was different among the groups (Fisher’s exact test p<0.001 for all comparisons). Normal nocturnal BP dipping was more frequent in controls (62%) compared to HF (28%) and LVAD patients (10%). Presence of reversed BP dipping profiles (for SBP and/or DBP) increased from controls (8%), to HF (16%), and LVAD (24%). Distribution of HR nocturnal dipping profiles is presented in Table 3B. Physiological HR reduction was more frequent in controls (59%) compared to HF (16%) and LVAD (14%) groups, while reverse nocturnal HR dipping occurred in the majority (52%) of LVAD patients (Pearson’s χ2 p<0.001 for all comparisons).
Table 3.
Dipping Profiles. (A) Distribution of blood pressure dipping profiles among the groups. (B) Distribution of heart rate dipping profiles among the groups.
| A) | |||
|---|---|---|---|
| Controls | Heart Failure | LVAD | |
| Both SBP & DBP nocturnal dipping, n (%) | 17 (62) | 7 (28) | 3 (10) |
| Only SBP nocturnal dipping, n (%) | 0 (0) | 0 (0) | 2 (7) |
| Only DBP nocturnal dipping, n (%) | 5 (19) | 7 (28) | 1 (4) |
| No nocturnal BP dipping, n (%) | 3 (11) | 7 (28) | 16 (55) |
| Both SBP & DBP nocturnal reverse dipping, n (%) | 0 (0) | 4 (16) | 2 (7) |
| Only SBP nocturnal reverse dipping, n (%) | 1 (4) | 0 (0) | 2 (7) |
| Only DBP nocturnal reverse dipping, n (%) | 1 (4) | 0 (0) | 3 (10) |
| B) | |||
| Controls | Heart Failure | LVAD | |
| HR nocturnal dipping, n (%) | 16 (59) | 4 (16) | 4 (14) |
| No HR nocturnal dipping, n (%) | 6 (22) | 15 (60) | 10 (34) |
| HR reverse nocturnal dipping, n (%) | 5 (19) | 6 (24) | 15 (52) |
DBP = Diastolic Blood Pressure; HR = Heart Rate; LVAD = Left Ventricle Assist Device; SBP = Systolic Blood Pressure.
Hypertension phenotypes
Overall, 24-hour BP monitor re-categorized 16 (20%) of the study subjects when compared to clinic visit: 3 (12%) controls, 6 (24%) HF and 7 (24%) LVAD patients. Table 4 shows the percent of patients with sustained normotension, sustained HTN, masked HTN, and white-coat HTN in each group. Distribution of hypertension phenotypes was different among the groups (Fisher’s exact test p=0.004 for all comparisons). Controls and HF patients had the highest proportion of sustained normotension. Sustained HTN was more frequent in LVAD (36%).White-coat HTN was more prevalent in LVAD (25%).
Table 4. Hypertension Phenotypes.
Distribution of hypertension phenotype profiles among the groups.
| Controls | Heart Failure | LVAD | |
|---|---|---|---|
| Normotensive, n (%) | 15 (56) | 18 (72) | 11 (39) |
| Sustained HTN, n (%) | 9 (33) | 1 (4) | 10 (36) |
| Masked HTN, n (%) | 2 (7) | 2 (8) | 0 (0) |
| White-Coat HTN, n (%) | 1 (4) | 4 (16) | 7 (25) |
HTN = Hypertension; LVAD = Left Ventricle Assist Device.
Discussion
The main findings of our study are the following: 1) LVAD patients exhibit abnormalities in circadian BP and HR variability that are similar to those observed in HF patients; 2) based on 24-hour monitoring, BP was not adequately controlled in 36% of LVAD patients; and 3) 25% of LVAD patients exhibited white-coat HTN.
Circadian changes in BP and HR have been extensively investigated in healthy and hypertensive subjects, with results indicating a higher risk of cardiovascular events in those with blunted or absent BP or HR reduction at night (18–23). In HF patients, a decrease in day-to-night variations in BP and HR correlates with disease severity (24, 25) and poor outcomes (5), likely reflecting enhanced autonomic (sympathetic) and neuro-hormonal activity in response to impaired cardiac function (26). Only one publication has described the circadian BP and HR patterns in LVAD patients. In 1994, Sehested et al. studied eight hospitalized HF patients who were supported with first-generation biventricular pulsatile pumps, showing that BP significantly declined during sleep, as did plasma norepinephrine and epinephrine (27). Since then, continuous-flow pumps have replaced first-generation pulsatile, volume displacement pumps. Our study is the first to describe 24-hour BP and HR profiles in patients on contemporary continuous flow LVAD support. Furthermore, differently from the prior study, we investigated outpatients rather than patients who were admitted to the hospital where sleep and activity routines are notoriously distorted.
In this study, we demonstrated that many LVAD patients have attenuated or reversed nocturnal reduction of BP despite mechanical circulatory support. Only 10% of LVAD recipients showed normal nocturnal BP dipping (compared to 62% of healthy controls and 28% of HF patients) and almost a quarter of them (24%) had reversed dipping profiles (compared to 8% of controls and 16% of HF). This abnormal nocturnal BP pattern may be explained by: i) a reduction in the normal increase in BP during day-time because of the inability of the LVAD to physiologically increase cardiac output during activities, and/or ii) failure to withdraw sympathetic nerve activity at night in these patients (28, 29). This latter mechanism may also explain why only 14% of LVAD patients had a nocturnal HR reduction (compared to 59% of healthy controls and 16% of HF patients). Notably, while HF and LVAD patients had the lowest MAP, 24-hour DBP values were higher in LVAD compared to HF, reflecting the unique hemodynamic profile of these pumps that flow continuously throughout the cardiac cycle, thus blunting BP decay during diastole (30). Whether these alterations in nocturnal dipping are associated with an increased risk of complication in LVAD patients is yet to be established.
Our study also provides evidence that 24-hour BP was controlled in less than 2/3 of LVAD patients. Elevated outpatient BP is associated with increased risk for stroke and pump thrombosis in HMII recipients(31). Discrepancies between clinic and ambulatory BP values were also frequent with recognition of white-coat HTN in several study subjects. Indeed, white-coat HTN was present in 25% of LVAD patients, similar to what was previously described in the general population (32). Implications of this finding in LVAD patients remain unclear. However, it is important to emphasize that patients with white-coat HTN are at risk of being overtreated (32, 33), possibly resulting in iatrogenic hypotension and even syncope. Consequences of these adverse effects are particularly relevant in LVAD, given that falls and head trauma can be catastrophic in patients who uniformly receive chronic anticoagulation based on the current guidelines. Thus, 24-hour ABPM may represent an important and more sensitive tool to guide therapy of antihypertensive drugs in this patient population.
Several limitations should be acknowledged. First, our analysis focused on the HMII and did not include the more recent HeartMate 3 (HM3) pump, since no ABPM monitors have been validated in LVADs that generate an “artificial pulse” (30). However, although the HMII and the HM3 are technically different, they are both continuous flow pumps and it is conceivable that their effects on circadian BP and HR variability are similar. Second, due to lack of established guidelines for the definition of HTN in the LVAD population, we arbitrarily utilized a MAP cutoff of 90 mmHg for the detection of elevated 24-hour ambulatory BP in these patients. Although no clinical validation of this cutoff values is currently available, it appears reasonable, at least for HM II, to use this value as it has been previously associated with thromboembolic complications (16, 34, 35). Third, in the absence of previous studies in LVAD, we arbitrarily utilized the same cut off that defines dippers in the normal population (>10% BP reduction at night), although we recognize that this threshold may overestimate the decrement in this metric. Fourth, the majority of LVAD patients (90%) were taking a beta blocker at the time of the study, and that could have blunted their HR circadian variability. However, a significant circadian variation in the HR was present in HF patients despite uniform use (100%) of beta blockers. Fifth, suboptimal control of blood pressure and reduced use renin angiotensin system blockers in in the LVAD group compared with the HF group (41% vs 88%) may have contributed to their altered circadian profile. Sixth, although the recruitment of controls and HF/LVAD patients from two different sites may have introduced bias, we tried to mitigate this risk by utilizing the same study protocol at both sites. Lastly, our results should not be considered conclusive given the small sample size and cross-sectional study design.
Twenty-four-hour BP and HR patterns are altered LVAD patients with marked attenuation of BP and HR dipping patterns at night. In addition, sustained and white-coat HTN are frequently identified in LVAD recipients. Larger prospective studies are warranted to validate our findings, investigate their prognostic implications and, eventually, test the impact of personalized management of BP and HR profiles in LVAD patients. Whether circadian modulation of pump speed might restore physiologic variability of BP and HR also remains to be established.
Supplementary Material
Acknowledgements
We thank Ms. Laura Watkeys and Dr. Maria Kearney (Cardiff Metropolitan University) for their assistance with the data collection.
Sources of Funding
Dr Castagna is supported by NIH grant T32HL144456.This research has been supported by a Research Grant from Abbott Laboratories and by funds from Lisa and Mark Schwartz Program to Reverse Heart Failure at New York-Presbyterian Hospital/Columbia University.
List of non-standard abbreviation:
- ABPM
Ambulatory blood pressure monitor
- DBP
Diastolic blood pressure
- HF
Heart failure
- HM II
HeartMate II
- HR
Heart rate
- HTN
Hypertension
- LVAD
Left ventricular assist device
- LVEF
Left ventricular ejection fraction
- MAP
Mean arterial pressure
- PI
Pulsatile index
- PP
Pulse pressure
- SBP
Systolic blood pressure
Footnotes
Disclosures
Dr. McDonnell received an unrestricted educational grant from IEM Healthcare in 2015.
Dr. Cockcroft received equipment and travel grants from IEM.
Dr. Colombo received research grants from Abbott Laboratories.
The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Gerhart-Hines Z, Lazar MA: Circadian metabolism in the light of evolution. Endocr Rev 2015;36:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parati G, Ochoa JE, Lombardi C, Bilo G: Assessment and management of blood-pressure variability. Nature reviews Cardiology 2013;10:143–55. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Ferrari A, Gregorini L, et al. : Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circulation research 1983;53:96–104. [DOI] [PubMed] [Google Scholar]
- 4.Bray MS, Young ME: Diurnal variations in myocardial metabolism. Cardiovasc Res 2008;79:22837. [DOI] [PubMed] [Google Scholar]
- 5.Shin J, Kline S, Moore M, et al. : Association of diurnal blood pressure pattern with risk of hospitalization or death in men with heart failure. Journal of cardiac failure 2007;13:656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casolo GC, Fazi A, Boddi M: Twenty-Four-Hour Heart Rate Behavior in Patients with Impaired Left Ventricular Function due to Coronary Heart Disease. Cardiology 1987;74:116–23. [DOI] [PubMed] [Google Scholar]
- 7.STEFENELLI T, BERGLER-KLEIN J, GLOBITS S, PACHER R, GLOGAR D: Heart rate behaviour at different stages of congestive heart failure. European heart journal 1992;13:902–7. [DOI] [PubMed] [Google Scholar]
- 8.Kario K: Nocturnal Hypertension: New Technology and Evidence. Hypertension 2018;71:9971009. [DOI] [PubMed] [Google Scholar]
- 9.Toschi-Dias E, Rondon MUPB, Cogliati C, Paolocci N, Tobaldini E, Montano N: Contribution of Autonomic Reflexes to the Hyperadrenergic State in Heart Failure. Front Neurosci 2017;11:162-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancini D, Colombo PC: Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. Journal of the American College of Cardiology 2015;65:2542–55. [DOI] [PubMed] [Google Scholar]
- 11.Parati G, Stergiou G, O’Brien E, et al. : European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. Journal of hypertension 2014;32:1359–66. [DOI] [PubMed] [Google Scholar]
- 12.Jones CR, Taylor K, Chowienczyk P, Poston L, Shennan AH: A validation of the Mobil O Graph (version 12) ambulatory blood pressure monitor. Blood pressure monitoring 2000;5:233–8. [DOI] [PubMed] [Google Scholar]
- 13.Luzardo L, Lujambio I, Sottolano M, et al. : 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertension research : official journal of the Japanese Society of Hypertension 2012;35:980–7. [DOI] [PubMed] [Google Scholar]
- 14.Castagna F, McDonnell BJ, Stohr EJ, et al. : Non-invasive measurement of peripheral, central and 24-hour blood pressure in patients with continuous-flow left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2017;36:694–7. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, et al. : 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). European heart journal 2018;39:3021–104. [DOI] [PubMed] [Google Scholar]
- 16.Bennett MK, Roberts CA, Dordunoo D, Shah A, Russell SD: Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2010;29:593–4. [DOI] [PubMed] [Google Scholar]
- 17.Teuteberg JJ, Slaughter MS, Rogers JG, et al. : The HVAD Left Ventricular Assist Device: Risk Factors for Neurological Events and Risk Mitigation Strategies. JACC Heart failure 2015;3:818–28. [DOI] [PubMed] [Google Scholar]
- 18.Salles GF, Reboldi G, Fagard RH, et al. : Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension 2016;67:693–700. [DOI] [PubMed] [Google Scholar]
- 19.Fagard RH, Celis H, Thijs L, et al. : Daytime and Nighttime Blood Pressure as Predictors of Death and Cause-Specific Cardiovascular Events in Hypertension. Hypertension 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi K, Hoshide S, Ishikawa J, et al. : Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. Journal of hypertension 2009;27:2265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA: Night day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. Journal of human hypertension 2009;23:645–53. [DOI] [PubMed] [Google Scholar]
- 22.Ohkubo T, Hozawa A, Yamaguchi J, et al. : Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. Journal of hypertension 2002;20:2183–9. [DOI] [PubMed] [Google Scholar]
- 23.Boggia J, Li Y, Thijs L, et al. : Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007;370:1219–29. [DOI] [PubMed] [Google Scholar]
- 24.van de Borne P, Abramowicz M, Degre S, Degaute JP: Effects of chronic congestive heart failure on 24-hour blood pressure and heart rate patterns: a hemodynamic approach. American heart journal 1992;123:998–1004. [DOI] [PubMed] [Google Scholar]
- 25.Caruana MP, Lahiri A, Cashman PM, Altman DG, Raftery EB: Effects of chronic congestive heart failure secondary to coronary artery disease on the circadian rhythm of blood pressure and heart rate. The American journal of cardiology 1988;62:755–9. [DOI] [PubMed] [Google Scholar]
- 26.Porter TR, Eckberg DL, Fritsch JM, et al. : Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. The Journal of clinical investigation 1990;85:1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehested J, Happe E, Ishino K, Hetzer R, Schiessler U, Schifter S: Diurnal variation in blood pressure in patients with biventricular assist devices and retained, nonpumping native hearts. Circulation 1994;89:2601–4. [DOI] [PubMed] [Google Scholar]
- 28.Cornwell WK 3rd, Tarumi T, Stickford A, et al. : Restoration of Pulsatile Flow Reduces Sympathetic Nerve Activity Among Individuals With Continuous-Flow Left Ventricular Assist Devices. Circulation 2015;132:2316–22. [DOI] [PubMed] [Google Scholar]
- 29.Heusser K, Wittkoepper J, Bara C, et al. : Sympathetic vasoconstrictor activity before and after left ventricular assist device implantation in patients with end-stage heart failure. European journal of heart failure 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castagna F, Stohr EJ, Pinsino A, et al. : The Unique Blood Pressures and Pulsatility of LVAD Patients: Current Challenges and Future Opportunities. Current hypertension reports 2017;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willey JZ, Boehme AK, Castagna F, et al. : Hypertension and Stroke in Patients with Left Ventricular Assist Devices (LVADs). Current hypertension reports 2016;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorostidi M, Vinyoles E, Banegas JR, de la Sierra A: Prevalence of white-coat and masked hypertension in national and international registries. Hypertension Research 2015;38:1–7. [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Thijs L, Hansen TW, O’Brein E, Staessen JA: White-Coat Hypertension. Hypertension 2013;62:982–7. [DOI] [PubMed] [Google Scholar]
- 34.Najjar SS, Slaughter MS, Pagani FD, et al. : An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2014;33:23–34. [DOI] [PubMed] [Google Scholar]
- 35.Pinsino A, Castagna F, Zuver AM, et al. : Prognostic implications of serial outpatient blood pressure measurements in patients with an axial continuous-flow left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2019;38:396–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


