Abstract
Background
Based on the new data from the primary analysis of the OPTIC (Optimizing Ponatinib Treatment in CP-CML) trial on dose optimization of ponatinib in patients with chronic phase (CP)-CML, the German consensus paper on ponatinib published in 2020 (Saussele S et al., Acta Haematol. 2020) has been updated in this addendum.
Summary
Focus is on the update of efficacy and safety of ponatinib, reflecting the new data set, as well as the update of the benefit-risk assessment and recommendations for ponatinib starting dose in CP-CML – provided that the decision to use ponatinib has already been made. Furthermore, based on OPTIC and additional empirical data, the expert panel collaborated to develop a decision tree for ponatinib dosing, specifically for intolerant and resistant patients. The recommendations on cardiovascular management have also been updated based on the most recent 2021 guidelines of the European Society of Cardiology (ESC) on cardiovascular disease prevention in clinical practice.
Key Messages
The OPTIC data confirm the high efficacy of ponatinib in patients with CP-CML and provide the basis for individualized dose adjustment during the course of treatment.
Keywords: Chronic myeloid leukemia, Ponatinib, Dosing regimens, Cardiovascular management, Consensus paper
Introduction
According to the ELN recommendations 2020, the third-generation tyrosine kinase inhibitor (3G TKI) ponatinib is currently the most potent TKI used in CML [1]. Ponatinib is effective in most clinically relevant BCR::ABL1 point mutations, including the T315I mutation [2]. Ponatinib is approved for the treatment of adults with CML in any phase of the disease who are resistant or intolerant to dasatinib or nilotinib, for whom subsequent treatment with imatinib is not clinically appropriate, or who have the T315I mutation [2]. Ponatinib is also approved for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) patients who are resistant or intolerant to dasatinib, for whom imatinib is not appropriate, or who are carrying the T315I mutation [2]. The final 5-year analysis of the pivotal phase II PACE trial demonstrated fast, deep, and durable responses in patients with heavily pretreated chronic phase (CP)-CML treated with ponatinib [3]. However, compared to imatinib, the more potent second- and third-generation TKIs are associated with a potential increase in cardiovascular (CV) risk to different extents, particularly arterial occlusive events (AOEs) [4]. However, each TKI may have a different cardiac and/or vascular toxicity profile in patients depending on their age, sex, comorbidities, and the presence of additional common CV risk factors (e.g., smoking, dyslipidemia, overweight, unhealthy lifestyle, diabetes mellitus) [5]. Differences in the inhibitory off-target effects of tyrosine kinases other than BCR::ABL1 may also determine their distinct toxicity profiles [5]. The newly approved STAMP (specifically targeting the ABL myristoyl pocket) inhibitor asciminib – which is indicated for the treatment of CP-CML after failure of two or more TKIs – appears to have a favorable CV safety profile [6]. Although only reported in a few cases, adverse CV events are still reported for asciminib in CML patients [6]. Thus, further evaluation of the potential CV adverse effects of asciminib is necessary.
Post hoc analyses of the PACE trial suggested that the CV risk with ponatinib is likely to be dose-dependent [7]. The PACE trial provided initial evidence that ponatinib dose reductions from the currently approved starting dose of 45 mg daily to 30 mg or 15 mg daily in patients with good response may reduce the incidence of these events while maintaining its efficacy in most patients [3]. The OPTIC trial evaluated a novel response-based dose reduction strategy to further understand the impact of ponatinib dose on safety and efficacy in patients with CP-CML resistant to a prior second-generation BCR::ABL1 TKI therapy or with a T315I mutation.
For the present addendum, two videoconferences were held with the authors to discuss and define the content based on the new publications. This was followed by the drafting of the manuscript, which was subsequently shared electronically several times with all authors for comments, additions, and suggested changes. The final manuscript was then read again by all authors and approved for submission.
OPTIC Trial: Optimal Dosing for Ponatinib in CP-CML
The randomized phase II OPTIC trial is prospectively evaluating the benefit-risk profile of three different ponatinib starting doses to develop a response-based dose reduction strategy in CP-CML patients with resistance or intolerance to at least 2 previous TKIs or with the T315I mutation [8]. 283 patients were randomized to a ponatinib starting dose of 45 mg, 30 mg, or 15 mg daily, with patients in the first two cohorts reducing their dose to 15 mg daily upon achieving a response ≤1% BCR::ABL1IS. The primary endpoint was the rate of ≤1% BCR::ABL1IS at 12 months, a target value that predicts a good long-term outcome [9]. The primary endpoint was reached when the ≤1% BCR::ABL1IS response rate was at least 35% with a lower limit of the confidence interval of 20%. A cross-cohort comparison was not statistically planned.
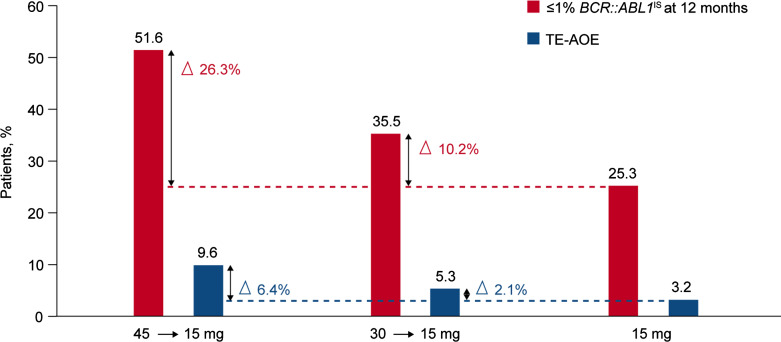
The ≤1% BCR::ABL1IS response rate at 12 months was 44.1% in the 45 mg cohort, 29% in the 30 mg cohort, and 23.1% in the 15 mg cohort; only the 45 mg cohort met the prespecified statistical endpoint. High cumulative rates of ≤1% BCR::ABL1IS were achieved in both patients with and without the T315I mutation at baseline (60% vs. 48.5% in the 45 mg cohort). Patients with a better than complete hematologic response to the previous TKI achieved high response rates both if they started with 45 mg and with 30 mg daily (50% and 58.6%, respectively). With a median follow-up of 32 months, the median overall survival (OS) was not reached in all cohorts. The estimated probability of OS at 24 months was more than 90% in the 3 cohorts. The median PFS was not reached in the 45 mg and 30 mg cohorts and was 45.6 months in the 15 mg cohort. Most patients were able to maintain their response after dose reduction to 15 mg upon achievement of response.
The rate of treatment-emergent AOEs was low (17 patients, 6% overall; 9.6% in the 45 mg cohort, 5.3% in the 30 mg cohort, and 3.2% in the 15 mg cohort), suggesting that response-based dose reduction may have contributed to the observed reduced risk of these events. Grade ≥3 AOEs occurred in 5.3% in the 45 mg cohort, 5.3% in the 30 mg cohort, and 3.2% in the 15 mg cohort. Treatment was discontinued in 3% due to AOEs. The most common nonhematologic adverse events were hypertension (28%), headache (18%), and increased lipase levels (17%), with similar rates across the three cohorts. The majority were graded as grade 1 or 2. The authors noted that these highly resistant CP-CML patients experienced clinical benefit from ponatinib across all cohorts. However, the highest benefit-risk profile was observed with the 45 mg starting dose of ponatinib reduced to 15 mg upon achievement of response (≤1% BCR::ABL1IS).
According to a propensity score analysis, the incidence of AOEs was substantially lower in OPTIC than in the PACE trial [10], keeping in mind that the OPTIC trial had a younger patient population (median age: 48 vs. 60 years in CP-CML patients in PACE) and a shorter median follow-up period compared to PACE (32 months vs. 56.8 months). In OPTIC, patients with well-controlled CVD at the time of study entry were eligible; 33% of the patients had at least one CV risk factor. In summary, the results of the OPTIC trial prospectively confirm that the safety and tolerability of ponatinib in the treatment of CP-CML can be optimized by a response-based dose reduction strategy without compromising clinical efficacy.
Benefit-Risk Assessment and Expert Recommendations for Ponatinib Starting Dose
Overall, the results of the OPTIC primary analysis suggest that the best benefit-risk profile for response-adapted dosing of ponatinib is achieved with the 45 mg starting dose with a dose reduction to 15 mg upon BCR::ABL1IS ≤1% is reached [8]. Figure 1 shows a descriptive clinical summary of overall safety and efficacy by ponatinib starting dose to illustrate the relationship between response and AOE rate.
Fig. 1.
Descriptive clinical summary of response rates and AOE rates by ponatinib starting dose in the OPTIC trial (adapted from [8]). TE-AOE, treatment-emergent arterial occlusive events.
Compared to the PACE trial, the dose reduction strategy in OPTIC resulted in a lower rate of CV events, a faster dose reduction, overall lower median relative dose intensity, fewer dose reductions due to side effects, and a longer median time on therapy [11].
Thus, the OPTIC trial confirmed the recommendation within the Iclusig® Summary of Product Characteristics (SmPC) [2] and the 2020 consensus paper [12] regarding the recommended ponatinib starting dose of 45 mg daily followed by dose reduction to 15 mg upon good response in the majority of resistant CP-CML patients, especially if BCR::ABL1IS was >10% (i.e., high leukemia burden) at therapy initiation [8]. However, the 45 mg starting dose may also be taken into consideration in patients without high-risk disease in order to achieve a higher response rate and faster response.
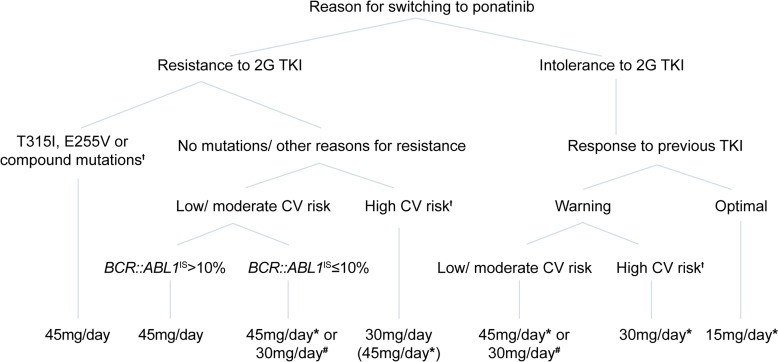
Based on current evidence and the experience of the authors in treating CML patients, a decision tree was developed jointly for the starting dose of ponatinib in CP-CML patients with resistance or intolerance to a 2G TKI (shown in Fig. 2). In the opinion of the expert panel, a lower starting dose of ponatinib may be considered in patients with high CV risk, lower leukemia burden (<10% BCR-ABL1IS) at therapy initiation, and/or in patients switching due to intolerance. The response-based dose reduction strategy of the OPTIC trial applies in principle to all patients for effective therapy with potentially fewer side effects. Most patients in OPTIC in whom the response was lost when the dose was reduced had the T315I mutation at baseline, and most were able to regain a response after reescalation of the dose [8]. Dose reescalation may therefore be an option for some patients and should be done as indicated in the SmPC [2].
Fig. 2.
Decision tree for recommended starting doses of ponatinib in patients with CP-CML. *The recommended starting dose of ponatinib is 45 mg/day, potentially reduced starting dose and dose modifications considering benefit-risk profile according to SmPC. †For patients with high and very high CV risk (according to SCORE2/SCORE2-OP or clinically manifest vascular disease), other therapeutic options should also be considered. #After careful consideration of the individual situation. 2G TKI, second-generation tyrosine kinase inhibitor; CV, cardiovascular. Warning defined according to the 2020 ELN recommendations (BCR::ABL1IS >10% at 3 months, 1–10% at 6 months, 0.1–1% at 12 months onward) [1]; optimal response defined according to the 2020 ELN recommendations (BCR::ABL1IS ≤10% at 3 months, ≤1% at 6 months, and ≤0.1% at 12 months onward) [1].
The ponatinib starting dose should be determined by evaluating each individual case, considering CML disease state and patient characteristics, prior treatment, mutation status, CV risk, and therapy goals. Moreover, the decision tree includes a risk stratification according to the dynamics of response over time [1]. Ponatinib should be started at 45 mg daily in patients with the T315I mutation, the E255V mutation, or a compound mutation [9]. A reduced starting dose of 30 mg is recommended in all patients with high or very high CVD risk (excluding patients with T315I, E255V, or a compound mutation) in both resistant and intolerant patients regardless of the reason for the switch to ponatinib. Starting with 30 mg ponatinib can also be considered in patients with low/moderate CVD risk who are intolerant to a 2G TKI and have a warning response to the previous TKI. Finally, in patients who have developed intolerance to previous TKIs but had already achieved at least MMR (≤0.1% BCR::ABL1IS), the initial ponatinib dose of 15 mg daily should always be considered in order to reduce potential drug-related risks. Table 1 provides a summary of the expert recommendations for ponatinib starting dose and dose adjustments in patients with CP-CML who are candidates for ponatinib.
Table 1.
Summary of the expert recommendations for ponatinib starting dose and dose adjustments in CP-CML patients who are candidates for ponatinib
| Ponatinib starting dose | Recommendations |
|---|---|
| 45 mg daily followed by dose reduction to 15 mg upon good response | In the majority of resistant CP-CML patients, especially with high leukemia burden (BCR::ABL1IS >10%) at therapy initiation [8] |
| In patients with T315I mutation, E255V mutation, or compound mutation [9] | |
| May be considered in patients without high-risk disease to achieve a faster response | |
| 30 mg daily | In patients with high/very high CV risk (excluding patients with T315I, E255V, or compound mutation) |
| In patients with lower leukemia burden (BCR-ABL1IS <10%) at therapy initiation | |
| In patients switching to ponatinib due to intolerance to a 2G TKI | |
| In patients with low/moderate CVD risk with intolerance to a 2G TKI and warning response to the previous TKI | |
| 15 mg daily | Should be considered in patients who have developed intolerance to previous TKIs but had already achieved at least MMR (BCR::ABL1IS ≤0.1%) |
| Ponatinib dose reescalation | May be considered in some patients who lost response after response-based dose reduction to possibly regain response [2] |
MATCHPOINT Trial: Ponatinib + FLAG-IDA in Blast Phase CML
Despite substantial improvements in the outcomes for patients with CP-CML, patients presenting with de novo blast phase (BP)-CML or progressing to BP from CP still have a significantly worse prognosis with a median OS less than 1 year after the diagnosis of BP [13]. The urgency of treatment of BP-CML is at the foreground as the long-term survival of patients with BP-CML depends on the achievement of a second CP followed by allogeneic stem cell transplantation (allo-SCT), as recommended by the European LeukemiaNet [1]. The multicenter phase I/II MATCHPOINT trial investigated whether the novel combination of ponatinib with FLAG-IDA chemotherapy (fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin) could improve response and optimize allo-SCT outcome in patients with BP-CML [14]. Overall, 17 patients were recruited, of which 16 were evaluable for the primary analysis. The median follow-up was 41 months. Using the EffTox model, the optimal dose of ponatinib in combination with FLAG-IDA was determined at 30 mg daily. 11 (69%) of 16 patients were in a second CP after one treatment cycle. 12 (71%) of 17 patients underwent allo-SCT. The most common grade 3/4 nonhematologic AEs were pulmonary infection, fever, and hypokalemia. There were 12 serious adverse events in 11 (65%) patients. 3 patients died from therapy-related AEs (cardiomyopathy, pulmonary hemorrhage, and bone marrow aplasia). Treatment-related mortality is within the expected range in this very high-risk patient group receiving intensive chemotherapy.
Management of BP-CML depends on the blast lineage (lymphoid or myeloid), prior (TKI) therapy, and resistance mutation profile. It has been shown that CML in blast crisis is associated with a poorer prognosis than de novo acute myeloid leukemia [13], indicating the need for an intensive chemotherapy regimen upfront. Several important factors, such as age, comorbidities, performance status, de novo versus progression on TKI, and mutational status, have to be considered prior to selecting therapy. The ELN currently recommends the use of a TKI in combination with acute myeloid leukemia chemotherapy regimens, such as FLAG-IDA and others, for the treatment of myeloid BP-CML [1]. The data from the MATCHPOINT trial suggest that ponatinib 30 mg plus FLAG-IDA can induce a second CP in patients with BP-CML and therefore may represent an active salvage therapy to bridge to allo-SCT.
CVD Risk Assessment and Management
The OPTIC trial provided substantial evidence that a response-based dose reduction strategy can improve the CV safety profile while maintaining its efficacy. Nevertheless, CV risk mitigation is an important part in the management of CML patients treated with TKIs. For the diagnosis and management of CV risk factors in CML and Ph+ ALL patients, the same guidelines and recommendations may be applied as for the general population as there are no data from large, prospective trials in CML or Ph+ ALL available. In this regard, the latest ESC Guidelines on cardiovascular disease (CVD) prevention in clinical practice 2021 [15] introduced new risk assessment scales with age-specific risk thresholds, some new risk factor treatment targets, and a stepwise treatment-intensification approach to achieve these targets. Apart from this, the recommendations for CV management from the 2020 consensus paper remain valid and apply in principle to all TKIs [12].
The ESC Prevention Guidelines 2021 aim for more personalized CVD prevention. Therefore, five groups were differentiated: people with CV risk factors but without CV events (referred as “apparently healthy people”), people with established atherosclerotic CVD, diabetes mellitus, familial hypercholesterolemia, and chronic kidney disease. The guidelines introduce new risk assessment scales which replace the previous SCORE scale with the SCORE2 and SCORE2-OP (older people) which apply to apparently healthy people to better estimate their CVD risk. SCORE2 applies to people under 70 years of age, and SCORE2-OP applies to those 70 years and older. Whereas the former SCORE estimated the risk of fatal CV events only, the SCORE2 and SCORE-OP estimate the 10-year risk of fatal and nonfatal CV events. In addition, both scales have been calibrated for the different European risk regions based on the WHO CV mortality rates. The guidelines also introduce age-specific risk thresholds for risk factor treatments in apparently healthy people, which are shown in Table 2. It is of note that the risk thresholds for considering treatment are lower for younger people, primarily to avoid undertreatment and increase benefit of lifelong risk factor treatment in this population. Although the guidelines recommend age-dependent risk stratification and age-specific risk thresholds, other factors such as lifetime CV risk, treatment benefit and harm, comorbidities, frailty, and patient preferences should be considered in all age groups when managing patients for primary CVD prevention. Since the exact mechanism with regard to CV events in the context of treatment with ponatinib has not yet been fully clarified, the decision of risk factor treatment in, for example, a patient <50 years of age with low CV risk according to SCORE2 is not supported by clinical evidence. However, as recent basic research data show that ponatinib leads to endothelial inflammation [16], it can be discussed in individual cases. Due to the anti-inflammatory effects of statins, the benefit of reducing CV events as well as the low rate of side effects, the generous use of this substance class also in low-risk patients may be considered. Moreover, we would recommend the initiation of therapy with Ca-antagonists (e.g., amlodipine) even in low-risk patients with high-normal blood pressure values due to the vasodilator component. Of course, this approach is a personal opinion and is not proven by large, randomized trials.
Table 2.
CVD risk categories based on SCORE2 and SCORE2-OP in apparently healthy people according to age (adapted from [15])
| CVD risk category | <50 years | 50–69 years | ≥70 years* | Treatment recommendation |
|---|---|---|---|---|
| Low-to-moderate | <2.5% | <5% | <7.5% | Risk factor treatment generally not recommended |
| High | 2.5–<7.5% | 5–<10% | 7.5–<15% | Risk factor treatment should be considered |
| Very high | ≥7.5% | ≥10% | ≥15% | Risk factor treatment generally recommended* |
CVD, cardiovascular disease.
*In apparently healthy people ≥70 years old, the treatment recommendation for lipid-lowering drugs is class IIb (“may be considered”).
In the ESC guidelines, the treatment targets and recommendations for patients with familial hypercholesterolemia, diabetes mellitus, and chronic kidney disease have remained largely the same. New in the lipid-lowering treatment is to add ezetimibe to statin therapy when LDL-C goals are not achieved with a statin at maximum tolerable dose. In patients with diabetes mellitus and additional coronary heart disease, the use of first-line drugs with proven CV benefits is recommended, such as the new class of sodium-glucose cotransporter 2 inhibitors (SGLT2). The treatment targets for systolic and diastolic blood pressure are now lower than in the previous guidelines for all patient groups, including older patients (systolic blood pressure <140 mm Hg or <130 mm Hg if tolerated, diastolic blood pressure <80 mm Hg for all treated patients). For full details on CVD risk stratification and treatment, please refer to the 2021 ESC Guidelines on CVD prevention in clinical practice, which can be found on the ESC website: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/2021-ESC-Guidelines-on-cardiovascular-disease-prevention-in-clinical-practice.
As with CV risk stratification in general (see above), CV monitoring during therapy with ponatinib and other TKIs requires an individualized approach. For ponatinib, two clinical trials with a thorough CV monitoring implemented are currently ongoing. The PONS trial (ClinicalTrials.gov Identifier: NCT03807479) is evaluating ponatinib as second-line treatment in CP-CML patients, who have failed previous TKI therapy. The PONTRACK trial (EudraCT number: 2018-004564-59) is assessing ponatinib in CML patients, who have not achieved a stable deep molecular response with nilotinib, dasatinib, and/or bosutinib after three or more years of TKI treatment.
Conclusion
The OPTIC trial represents the first study that prospectively evaluated a response-based dose reduction strategy to optimize the benefit-risk profile of a TKI in patients with CP-CML [8]. Data from the OPTIC primary analysis demonstrated the high efficiency of ponatinib while considerably improving CV risk due to the response-based dose reduction. Based on the currently available evidence and experience of the experts in the treatment of CML, a decision tree for the starting dose of ponatinib in CP-CML patients with resistance or intolerance to a 2G TKI was jointly developed. The main novelties of the 2021 ESC Guidelines on CVD Prevention are also summarized, including the new CVD risk assessment charts SCORE2 and SCORE2-OP [15]. Finally, based on the findings of the MATCHPOINT trial, the combination of ponatinib and FLAG-IDA might indicate a potentially important advance in the treatment of blast phase CML, which currently has a very poor outcome [14].
Conflict of Interest Statement
Susanne Saussele has received research funding from Novartis, Bristol-Myers Squibb, and Incyte and honoraria from Bristol-Myers Squibb, Incyte, Novartis, Pfizer, and Roche. Paul La Rosée is an advisory board member for Novartis, Bristol-Myers Squibb, and Incyte and has received research funding and speaker honoraria from Novartis. Alexander Kiani has received honoraria for lectures and consulting services from Novartis, Bristol-Myers Squibb, and Pfizer. Wilhelm Haverkamp has received honoraria for lectures from Abbott, ARIAD, Amicus, ASTRA, Bayer, Daiichi Sankyo, Novartis, Pfizer, and Sanofi. Kathleen Jentsch-Ullrich has no conflicts of interest to declare. Frank Stegelmann reports honoraria from and/or consultancy for AOP Pharma, Bristol-Myers Squibb/Celgene, Incyte, Novartis, and Pfizer. Christina Rieger received speaker honoraria from and served as a consultant to AbbVie, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Incyte, Ipsen, Janssen Cilag, MSD, Novartis, and Sanofi Pasteur. Cornelius F. Waller reports honoraria for lectures from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, MSD, Novartis, Pfizer, Roche, and Takeda; support for attending meetings and/or travel from Bristol-Myers Squibb and MSD; honoraria for advisory board services from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and MSD; and consulting fees from Viatris, Roche, and Alvotech. Georg-Nikolaus Franke reports honorary/advisory board services for Incyte, Pfizer, MSD, Jazz, and Novartis and has received travel support from Gilead/Kite and Takeda. Christian Junghanss reports honoraria and research grants from Incyte, Amgen, and Novartis. Rudolf Kirchmair has received a research grant from ARIAD. Markus Theurl participated in a basic research project partially funded by ARIAD. Philipp le Coutre reports honoraria from Incyte, Novartis, and Pfizer.
Funding Sources
Medical writing assistance was provided by Gerhard Emrich and funded by Incyte Corporation.
Author Contributions
Conceptualization, writing – review and editing, and approval of the final manuscript: Susanne Saussele, Paul La Rosée, Alexander Kiani, Wilhelm Haverkamp, Kathleen Jentsch-Ullrich, Frank Stegelmann, Christina Rieger, Cornelius F. Waller, Georg-Nikolaus Franke, Christian Junghanss, Rudolf Kirchmair, Markus Theurl, and Philipp le Coutre; writing – original draft: Susanne Saussele, Philipp le Coutre, Wilhelm Haverkamp, Rudolf Kirchmair, and Markus Theurl.
Funding Statement
Medical writing assistance was provided by Gerhard Emrich and funded by Incyte Corporation.
References
- 1. Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Medicines Agency . Iclusig (ponatinib). Summary of Product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/iclusig-epar-product-information_en.pdf. [Google Scholar]
- 3. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR::ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic Review and meta-analysis. JAMA Oncol. 2016;2(5):625–32. [DOI] [PubMed] [Google Scholar]
- 5. Lopina N, Dmytrenko I, Hamov D, Lopin D, Dyagil I. A new paradigm of cardio-hematological monitoring in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Cureus. 2022;14(6):e25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, versus bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84–91. [DOI] [PubMed] [Google Scholar]
- 8. Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):2042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hochhaus A, Breccia M, Saglio G, García-Gutiérrez V, Réa D, Janssen J, et al. Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. 2020;34(6):1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kantarjian HM, Deininger MW, Abruzzese E, Apperley J, Cortes JE, Chuah C, et al. Efficacy and safety of ponatinib (PON) in patients with chronic-phase chronic myeloid leukemia (CP-CML) who failed one or more second-generation (2G) tyrosine kinase inhibitors (TKIs): analyses based on PACE and OPTIC. ASH Ann Meet. 2020;136:43–4. [Google Scholar]
- 11. Jabbour EJ, Deininger MW, Abruzzese E, Apperley JF, Cortes JE, Chuah C, et al. Dose modification dynamics of ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) from the PACE and Optic trials. Blood. 2021;138(Supplement 1):2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saussele S, Haverkamp W, Lang F, Koschmieder S, Kiani A, Jentsch-Ullrich K, et al. Ponatinib in the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute leukemia: recommendations of a German expert consensus panel with Focus on cardiovascular management. Acta Haematol. 2020;143(3):217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hehlmann R, Saußele S, Voskanyan A, Silver RT. Management of CML-blast crisis. Best Pract Res Clin Haematol. 2016;29(3):295–307. [DOI] [PubMed] [Google Scholar]
- 14. Copland M, Slade D, McIlroy G, Horne G, Byrne JL, Rothwell K, et al. Ponatinib with fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor chemotherapy for patients with blast-phase chronic myeloid leukaemia (MATCHPOINT): a single-arm, multicentre, phase 1/2 trial. Lancet Haematol. 2022;9(2):e121–32. [DOI] [PubMed] [Google Scholar]
- 15. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337. [DOI] [PubMed] [Google Scholar]
- 16. Paez-Mayorga J, Chen AL, Kotla S, Tao Y, Abe RJ, He ED, et al. Ponatinib activates an inflammatory response in endothelial cells via ERK5 SUMOylation. Front Cardiovasc Med. 2018 Sep 6;5:125. [DOI] [PMC free article] [PubMed] [Google Scholar]