Fig. 1.
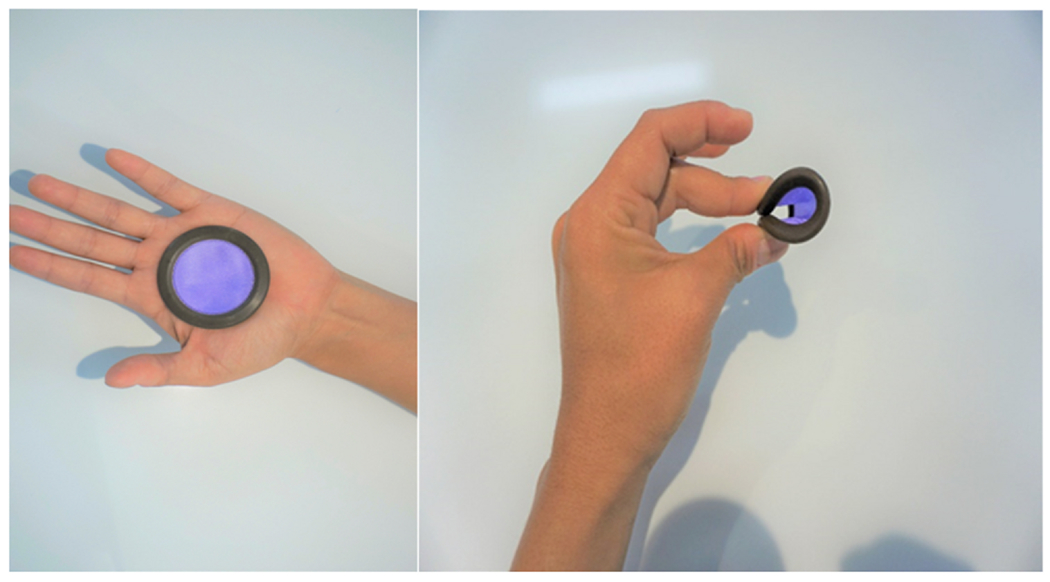
Ovaprene, an investigational vaginal contraceptive evaluated in a 2019 United States multicenter study. Ovaprene consists of a 55 mm silicone ring with a central permeable barrier. The barrier’s pore size inhibits movement of sperm while allowing passage of fluids. Ferrous gluconate released from the ring causes oxidative damage to the lipid bilayer of the sperm tail, leading to spermiostasis. Ascorbic acid is released to maintain ferrous gluconate in its ferrous state. Unlike other vaginal barrier methods, the Ovaprene is inserted at the end of one menstrual period and left until the beginning of the next, requiring no action at intercourse. It requires no clinician fitting, and a new product is used each month.
