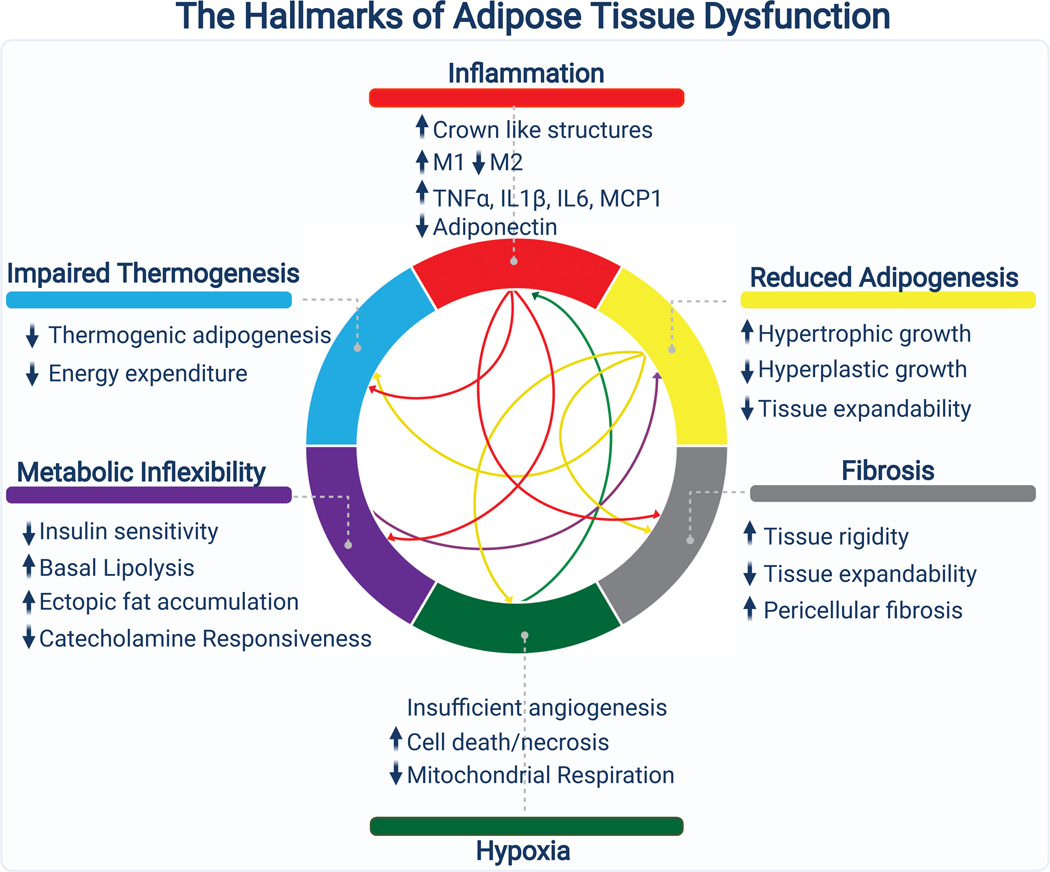
Figure 6. The Hallmarks of Adipose Tissue Dysfunction.
Several interconnected and mutually reinforcing processes contribute to adipose tissue dysfunction during the pathogenesis of metabolic disease. Excessive expansion of adipose tissue and insufficient angiogenesis drives hypoxia, which triggers an inflammatory response and promotes fibrosis. The inflammatory milieu drives the secretion of cytokines that maintain adipose inflammation, promote fibrosis, and impair metabolic flexibility by interfering with both nutrient uptake and nutrient release. Diminished progenitor cell differentiation capacity, due to progenitor autonomous defects, inflammation, or fibrotic ECM, limits expansion via hyperplasia, favoring adipocyte hypertrophy. Thermogenesis, which is highly dependent on the metabolic flexibility of adipose tissue is blunted, diminishing energy expenditure. These processes are continuous and synergistic, leading to a vicious cycle that culminates in adipose tissue dysfunction.