Abstract
The migration of metal ions to the food matrix has been always a challenge in the production of active food packaging films. In this study, it was tried to evaluate the idea of using hairy cellulose nanocrystals (HCNs) in controlling the migration of Silver Nanoparticles (AgNPs) from polycaprolactone (PCL)-based films to the Tilapia fish. HCNs and the final films (integrated with various amounts of HCNs and AgNPs) were evaluated physicochemically and mechanically. Tilapia fish were packed using the films and after specific periods, the fish samples were assessed microbiologically and physiochemically. According to the results, incorporating NPs into PCL films enhanced tensile strength, elasticity, and toughness making the films more resistant to breakage and deformation under stress. The introduction of HCNs reduced the surface roughness level, decreasing AgNPs migration, but also accelerated the degradation rate. Films with [1% AgNPs +2% HCNs] and [1% AgNPs] had the lowest and highest water vapor transmission rate. The use of AgNPs (1%) + HCNs (2%) incorporated into PCL films resulted in a lower pH value, TVB-N, TBARs, and PV. It also decreased microbial activities in samples in comparison to the control. Therefore, the idea of using HCNs along with antibacterial metal-based nanoparticles can control the rate of ion migration.
Keywords: Polycaprolactone film, Hairy cellulose nanocrystals, Silver nanoparticles
Highlights
-
•
HCN clumping, uncovering a diameter increase from 98 nm to 823 nm, providing new insights into structural characteristics
-
•
SEM images confirm well-defined rod-like and spherical nanosized cellulose particles, validating the successful synthesis of HCNs with uniform and dispersed morphology.
-
•
Incorporating HCNs in films enhances their biodegradability with an effective degradation rate, offering valuable insights into this field.
-
•
The synergistic antimicrobial effects of Ag NPs and HCNs in SA films results in microbial activity reductions and Tilapia meat shelf-life extension.
1. Introduction
Tilapia (Oreochromis niloticus), due to its high nutritional value (rich in unsaturated fatty acids, essential amino acids, minerals, and vitamins), low price, few bones, and attractive flavor, is widely consumed or utilized to produce high-quality salt fish and ready-to-eat fish products. However, due to microorganism proliferation, protein degradation, oxidation, and drip loss, it is susceptible to deterioration during shelf life. Packaging is one of the best techniques to inhibit decreased fish quality during storage time. Plastic packaging films are problematic due to poor sterilization, antibacterial performance, and environmental pollution (Kavuncuoglu, Yalcin, & Dogan, 2023; Zeng, Yang, Tong, & Zhao, 2023). Hence, using safe and green biodegradable biopolymers incorporated with antimicrobial ingredients (active packaging) has become the main focus of food packaging research (Mukhametov, Shamekova, Dautkanova, Kazhymurat, & Ilyassova, 2023; Yu, Fei, He, & Li, 2021; Zhang et al., 2023).
While only a limited number of synthetic polymers exhibit the crucial trait of biodegradability, polycaprolactone (PCL) has garnered increased attention in recent times due to its biocompatibility and biodegradability. This polymeric material shares similarities with conventional plastics while possessing the added advantage of being biodegradable. This characteristic opens up avenues for replacing non-biodegradable synthetic plastics commonly used in everyday items such as bags, packaging, and bottles (Sadeghi, Razavi, & Shahrampour, 2022). In the food industry, PCL is fashioned into porous or dense films to accommodate bioactive compounds in packaging design, thereby prolonging the shelf life of food items. Concerning encapsulation applications, PCL serves to shield bioactive compounds, enabling their incorporation into food products and enhancing their nutritional value. In agro-industrial settings, PCL microcapsules safeguard target substances from degradation, ensuring their stability until they are delivered to plants or soils (Oney-Montalvo, Dzib-Cauich, de Jesús Ramírez-Rivera, Cabal-Prieto, & Can-Herrera, 2024).
Hairy nanocellulose, recently introduced, is a novel type of nanocellulose in which amorphous cellulose chains protrude from both ends of a crystalline body (Van De Ven & Sheikhi, 2016). These cellulose protrusions, or fiber extensions, are inherently rich in bi-functional aldehyde-carboxylic acid moieties, aldehyde groups in sterically stabilized nanocrystalline cellulose, and carboxylic acid groups in electro-sterically stabilized nanocrystalline cellulose. The hairs consist of dialdehyde and carboxylated containing disordered cellulose chains, rendering high chemical reactivity and colloidal stability. The most functional groups are located in the amorphous regions, and these accessible regions can be extensively and easily modified with various functional groups (Tavakolian et al., 2019).
Silver nanoparticles (AgNPs) are one of the most used nanomaterials in food packaging due to their antibacterial properties. AgNPs possess antimicrobial, antifungal, anti-yeast, and antiviral properties, making them suitable for integration into active food packaging alongside both non-biodegradable and edible polymers (Khursheed et al., 2023). AgNPs can be mixed with various matrices, like stabilizing agents, polymers, and biodegradable and non-biodegradable compounds. Excellent antibacterial properties of packaging containing AgNPs contribute considerably to enhanced shelf life (Onyeaka, Passaretti, Miri, & Al-Sharify, 2022). High antimicrobial attributes of AgNPs have been reported in chicken meat (Majumder, Huang, Zhou, Wang, & George, 2023). AgNPs have been widely used as an antimicrobial agent in food packaging technologies. However, the risks associated with their potential migration into foods are a major concern as it has implications for human health associated with their toxicity properties. Addressing this challenge requires the exploration of novel approaches to mitigate ion migration, potentially involving the incorporation of additives or surface modifications aimed at impeding ion mobility (Carbone, Donia, Sabbatella, & Antiochia, 2016).
By strategically enhancing the packaging matrix to regulate ion diffusion, advancements can be made toward the development of more effective and reliable silver-based active packaging solutions for ensuring food quality and safety. In this study, it was tried to evaluate the idea of using HCNs along with AgNPs to develop a PCL-based active food packaging with reduced Ag migration. To achieve this, at first, the HCNs were synthesized and evaluated physiochemically, and then PCL-based films were fabricated containing various amounts of HCNs and AgNPs. The final films were studied and used to package Tilapia fish.
2. Materials and methods
2.1. Preparation of nanocomposite films
Synthesis of HCNs: This step was performed according to the study by Esmaeili, Pirzadeh, Pakrooyan, Lukolayeh, and Kırboğa (2024) with a little modification (Esmaeili et al., 2024). Spinach samples were washed, swollen in deionized water (24 h), and bleached in sodium hydroxide (NaOH, Merck) (3% w/v) and Sodium hypochlorite (NaOCl, Merck) (5% w/v) solutions (100 mL, 60 °C, 5 days). The spinach-bleached stems were decellularized, stored in deionized water (4 °C), and pulverized in water.
The decellularized Spinach leaf (10 g) was oxidized with Sodium chloride (NaCl, Merck) (38.7 g), Sodium periodate (NaIO4, Merck) (13.2 g), and distilled water (625 mL) in the dark (42 h) at room temperature. Ethylene glycol (10 mL) quenched the reactions, and the fibers were filtered using a 4 μm filter, washed, and dehydrated with ethyl alcohol under slow agitation (3 times, 10 h). The filaments named HCNs were immersed in alcohol (24 h, 25 °C). For amination, HCN (4 g), ammonia bicarbonate (2.5 g), and ammonium (80 m mol) were added to a beaker containing 200 mL of ethanol to reduce the amine group at 80 °C. Finally, the reaction was stopped by placing the beaker in an ice bath, and then 0.46 g of NaBH4 was slowly added to the mixture and stirred gently on a stirrer for one hour. The final HCNs were centrifuged and stored at 4 °C.
Preparing the polymeric films: Polycaprolactone (Sigma-Aldrich) (0.5 g) was dissolved in chloroform (Merck) (10 mL) and mixed with the appropriate amount of AgNPs)ARMINANO Co., Cas No. 7440-22-4) and HCNs for two hours (Yu et al., 2021). The final solutions were cast and left at room temperature for 7 days to evaporate the chloroform. Then the films were washed with sterile distilled water several times. Four polymeric films were fabricated with the concentration of AgNPS and HCNs including film A (1% w/w AgNPs), film B (1% w/w AgNPs and 1% w/w HCNs), film C (1% w/w AgNPs and 2% w/w HCNs), Film D (1% w/w HCNs), and control.
2.2. Film properties
2.2.1. Scanning Electron microscopy (SEM)
The surface morphology of films was studied by scanning electron microscopy (AIS 2000 model, Seron Technology, Korea) (Yu et al., 2021).
2.2.2. Energy dispersive X-ray (EDX) analysis
The crystal structure of the HCNs was analyzed by EDX (Mahamax, Iran). A speed of 40/min was used for recording EDX patterns (Balakrishnan et al., 2019).
2.2.3. Dynamic light scattering (DLS) and zeta potential
The DLS method was used to assess the particle size distribution and mean diameter of NPs using a Shimadzu particle size analyzer (SALD 2101, Japan). Before performing size measurement (at 25 °C), distilled water was used to dilute the samples (1:100) to avoid multiple laser dispersion caused by the particle accumulation. The zeta potential of NPs was assessed at 25 °C by a Zetasizer Nano (ZS90, Malvern, U.K.) based on Dilbaghi, Kaur, Ahuja, Arora, and Kumar (2014).
2.2.4. Fourier transform infrared spectroscopic (FTIR) analysis
The structural attributes of nanoparticles were assessed at 25 °C by FTIR spectra (Bruker Co., Germany) based on the method reported by Balakrishnan et al. (2019).
2.2.5. Atomic force microscopy (AFM)
An APER-A-100 SPM was used to evaluate the sample AFM based on Balakrishnan et al. (2019).
2.2.6. Mechanical properties
The elongation at break and tensile strength (TS) of the meat samples were determined based on the ASTM 882–00 standard (ASTM, 2001) using an Instron 5543 Co. (Canton, MA, USA) apparatus. Tensile strength (TS) was calculated based on methods reported by Yu et al. (2021).
2.2.7. Biodegradability analysis
To evaluate the films' biodegradability, agricultural soil (50 g) and water (10 mL) were added to plates along with a piece of film (1 × 1 cm). Water (5 mL) was sprayed on it every three days. The samples were evaluated after 1, 7, 14, and 21 days. The samples were cleaned, dried for 24 h at 50 °C, and weighed. Eq. 1 was used for determining weight loss:
| (1) |
where Mf and Mi denoted final mass and initial mass, respectively (Balakrishnan et al., 2019).
2.2.8. Water vapor transmission rate (WVTR)
The weight reduction was calculated by a microbalance of gravimetric apparatus (IGA-003). The slope of weight change versus time was used to obtain the WVTR values by the exposed surface area of samples (Yu et al., 2021) (Eq. 2).
| (2) |
2.3. Chemical and microbiological evaluation of samples
The evaluation was performed in 3 runs over 3 days 0, 3, and 7. Meats were washed, sized (1 × 2 × 3 cm3), and packed by films containing various percentages of AgNPs and/or HCNs according to the polymer weight. The experimental procedures were conducted in triplicate, and control samples were included.
2.3.1. pH values
pH value of the samples was assessed using a pH meter (Hanna, Methrom, Switzerland) after homogenization of samples (1:10 v/v) with distilled water (Afrin et al., 2023).
2.3.2. Peroxide value (PV)
For the measurement of peroxide value, fish oil (1 g), potassium iodide (1 g), acetic acid (20 mL), and chloroform were mixed and heated (30 s) using a boiling water beaker. Then, starch (1–5%) and potassium iodide solution (20 cm3, 5%) were mixed, and added sodium hyposulfite solution 1.5 N for titration (Afrin et al., 2023). The content of peroxide value expressed in milli-eq of oxygen/sample (kg) in Eq.3:
| (3) |
2.3.3. Thiobarbituric acid reaction (TBARs)
The determination of TBARS values in tilapia samples followed the methodology described by Yaghoubi et al. (2021). The oxidation products were assessed through their reaction with thiobarbituric acid, with subsequent quantification performed using a spectrophotometer (UNICO, US) set at a wavelength of 532 nm. Standard curves were prepared using 1,1,3,3-tetraethoxypropane (TEP) across concentrations ranging from 0 to 10 ppm, facilitating the expression of data in terms of mg of malondialdehyde per kilogram (mg MDA/kg) of chicken meat samples.
2.3.4. Total volatile basic nitrogen (TVB-N)
The assessment of total volatile nitrogen (TVB-N) in meat samples was conducted utilizing the Kjeldahl method, employing vapor distillation following the procedure outlined by Yaghoubi et al. (2021). The resulting data were expressed in milligrams per 100 g (mg/100 g) of Tilapia meat samples.
2.3.5. Microbial activity
25 g of meat samples were homogenized with 225 mL of peptone water (0.1%) by sterile lab blender (Neutec; Paddle Lab Blender, USA) for 3 min. Serial dilutions were prepared with peptone water (0.1% w/v; Difco, Becton Dickinson). Plate count agar was used to evaluate total viable counts (PC Agar, Merck; 48–72 h at 30 °C and seven days at 7 °C for total thermophilic and psychrotroph count, respectively). Staphylococcus aureus and coliforms were enumerated on Baird Parker agar (48 h at 30 °C) and Red bile agar (VRB Agar, Merk; 24 h at 37 °C), respectively. The pour plate method was utilized for bacteria enumeration. The results of bacterial count were reported as Log CFU/g of meat samples (Zhai, Zhou, Zhang, Wang, & Hou, 2022).
2.4. Evaluation of nanoparticle migration into Tilapia tissue
1 g of Tilapia sample, packed in nano-film, was heated at 500 °C for 1 h using an oven. All the elements in the sample were evaporated and destroyed, and only silver material remained. After reaching ambient temperature, 20 mL of nitric acid (1 N) was added, forming nitrate salt. The metal was separated from the bottom of the container and became metal salts that can be dissolved in water. The obtained liquid was diluted to 50 mL. The amount of nanoparticle migration to the sample was measured by the ICP-MS apparatus (Liu et al., 2019).
2.5. Statistical analysis
The statistical analysis of fish sample results was assessed using statistical analysis software (SAS) (v.9, SAS Institute, United States America). A random block design was used to analyze the results using a mixed impact of storage period (7 days), sample treatments as fixed effects, and 3 measurements as a random effect. Statistical significance was shown by p ≤ 0.05; all results were indicated as mean values ± SE.
3. Results and discussion
3.1. HCNs characterization
The HCNs characterization including DLS analysis, zeta potential, SEM images, and FTIR were shown in Fig. 1. The size of HCNs was detected using DLS analysis. Based on the obtained results, two peaks can be observed: the first peak indicated the presence of 92.96% of NPs with a diameter of 98 nm, and the second peak revealed the presence of approximately 3% of NPs with a diameter of 823 nm. It can be concluded that the reason for the increase of the NPs diameter from 98 nm to approximately 823 nm might be attributed to clumping (aggregation of NPs and their connection) (Fig. 1). The hydrodynamic diameter of HCNs can be in wide ranges from 82 nm up to 150 nm, or 5–150 nm (Koshani, Eiyegbenin, Wang, & van de Ven, 2022; Koshani, Zhang, van de Ven, Lu, & Wang, 2021). Homogenization and enzymatic/acid hydrolysis cause the breaking of hydrogen bonds and cleaving of cellulose chains to produce nano-cellulose. Based on the technique, the surface charge of HCNs may be positive, negative, or neutral (Van De Ven & Sheikhi, 2016). The main reason might be related to both crystalline and amorphous regions, which, based on the synthesis protocol, the cleavage may happen in the amorphous site.
Fig. 1.
The HCNs characterization (a: DLS analysis, b: zeta potential, c: SEM images, and d: FTIR) and the SEM of prepared films containing A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs.
Three peaks with different zeta potentials have been obtained (Fig. 1a). The first peak confirmed that nearly 21% of HCNs had a zeta potential of around −40.3 mV. About 25% of HCNs showed a positive zeta potential of 27.9 mV, and 15.3% of HCNs showed a zeta potential of +134 mV. The concentration of NPs is one of the most critical factors in evaluating the zeta potential. So, the accumulation of NPs directly affects the value of the zeta potential. By the evolution of the obtained peaks (Fig. 1b), helpful information regarding the structure of the produced NPs can be obtained. The nanoparticle structure has a negatively charged part (amorphous part) and a positively charged part (crystalline part). Cellulose chains, composed of laminated fibers resembling small hairs, are a common feature of crystalline segments. These chains, which protrude from the poles of the crystalline segments, are not soluble in isopropanol. As a result, the chains remain intact even when exposed to this solvent. This is because the isopropanol molecules cannot break down the deaminated fibers of the chains. Therefore, the chains remain protruding from the poles of the crystalline segments despite exposure to isopropanol (Koshani et al., 2022).
Results of SEM images demonstrated that the developed HCNs had a well-defined morphology and were in the shape of rod-like and spherical nanosized cellulose particles (Fig. 1c). The spherical-like and rod-like NPs were found to have an average length and diameter of <200 nm. However, some spherical NPs had an average diameter of about 20 nm. According to the DLS results, these particle sizes are consistent with the dimensions of aminated nanocrystalline cellulose particles. The synthesized particles were uniform and well-dispersed, although accumulation was observed, which might be related to the sample preparation for SEM analysis. Similar results were reported by Koshani et al. (2021).
The outcomes of the FTIR analysis are depicted in Fig. 1d. The peak that appeared at 616.73 cm−1 is attributed to the O—H bending. The peak that appeared at 931.13 cm−1 can be justified by the deformation of the C—H bond of the glucosidic bond between the glucose units. Peaks at 1238.03 cm−1 and 1315.31 cm−1 were due to the bridge oxygen stretching and CH2 wagging, respectively (Mahrous, Koshani, Tavakolian, Conley, & van de Ven, 2021; Morantes, Muñoz, Kam, & Shoseyov, 2019). The broad peak at 3435.32 cm−1 was related to the stretching of the hydroxyl group. As a result, it is difficult to detect the amine groups; instead, HCNs showed a prominent sharp peak for the C-NH2 bending vibrations at 1635 cm−1. The characteristic peak of aldehyde groups around 931.13 cm−1 implies the conversion of the aldehyde to amine groups (Ly, Thielemans, Dufresne, Chaussy, & Belgacem, 2008).
3.2. Physical and chemical characterization of the fabricated films
3.2.1. SEM and EDX
Several parameters that can affect the quality of these films are as follows: the thickness of the film, the uniformity of the film, the materials used to form the film, and the surface properties of the film.
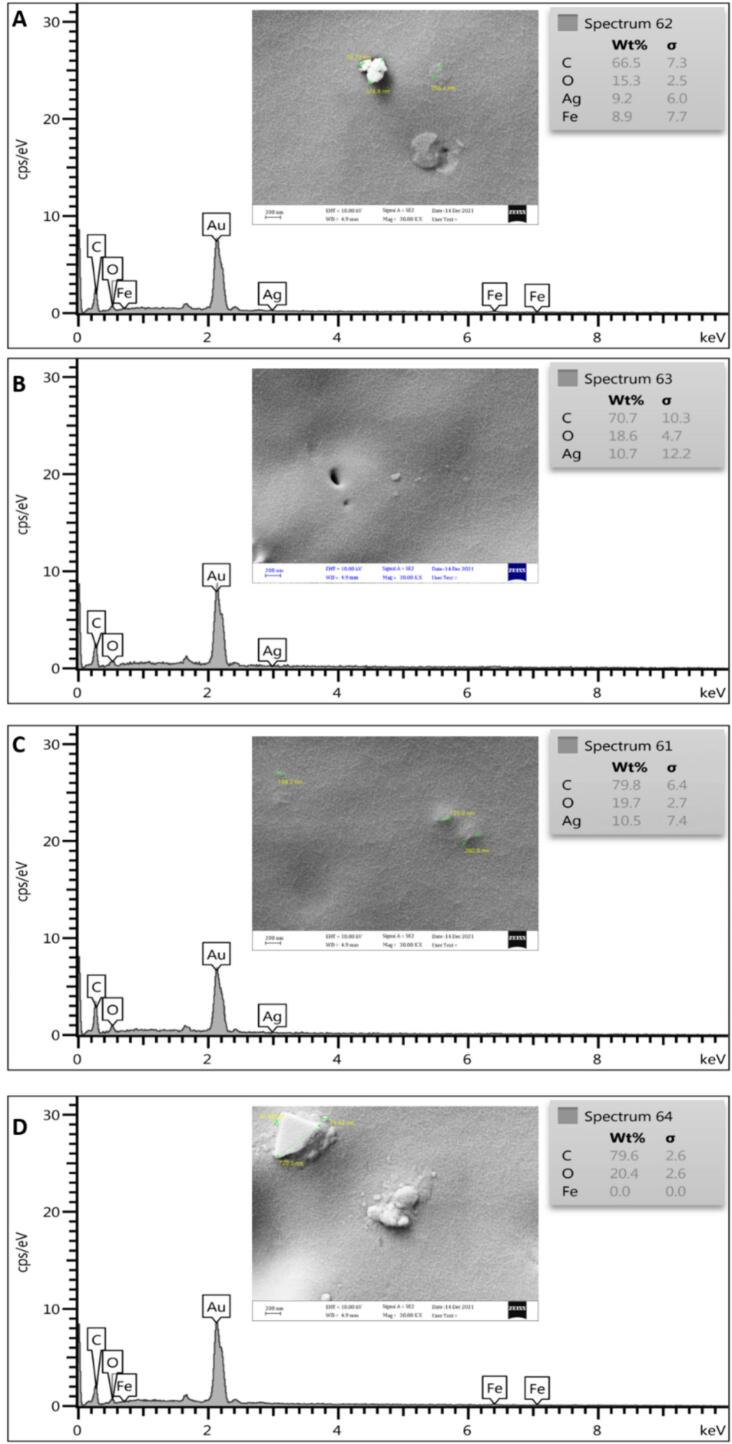
The morphological attributes of the prepared films were studied using SEM (Fig. 1A-D) and EDX (Fig. 2A-D). The SEM results showed the presence of uniform surfaces in all of the prepared films. The lumping phenomenon can be observed on the surface of the films, indicating unevenness. Due to the lack of roughness, it can be concluded that HCNs were well dispersed and fixed inside the film structure. It can minimize the risk of high WVTR and low mechanical strength for food packaging approaches. However, several parameters (e.g., film concentration, drying rate, HCNs dispersion) must be considered during film preparation to reach a uniform film.
Fig. 2.
EDX analysis of prepared films containing A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs.
The EDX results obtained from the films proved that there were no silver NPs in group C films, while in groups A and B, the amount of silver NPs was close to each other and equal to 9.2 and 10.7, respectively. From the SEM and EDX results, it can be concluded that the concentration of silver NPs and the uniformity of silver NPs per unit area of the produced films are the same.
Combining SEM and EDX results, it can be inferred that AgNP concentration and uniformity per unit area are consistent across the produced films. Recent studies have emphasized the critical role of NP dispersion and distribution within polymer matrices in determining barrier properties and antimicrobial efficacy in nanocomposite films. Additionally, the interaction between nanoparticle morphology and film surface properties has been shown to affect water vapor barrier performance, highlighting the importance of optimizing film composition and processing parameters. By elucidating these relationships, a more comprehensive understanding of the structure-property-function relationships of the fabricated films can be achieved, informing future advancements in food packaging technology (Attaran, Hassan, & Wahit, 2017).
3.2.2. FT-IR and mechanical properties
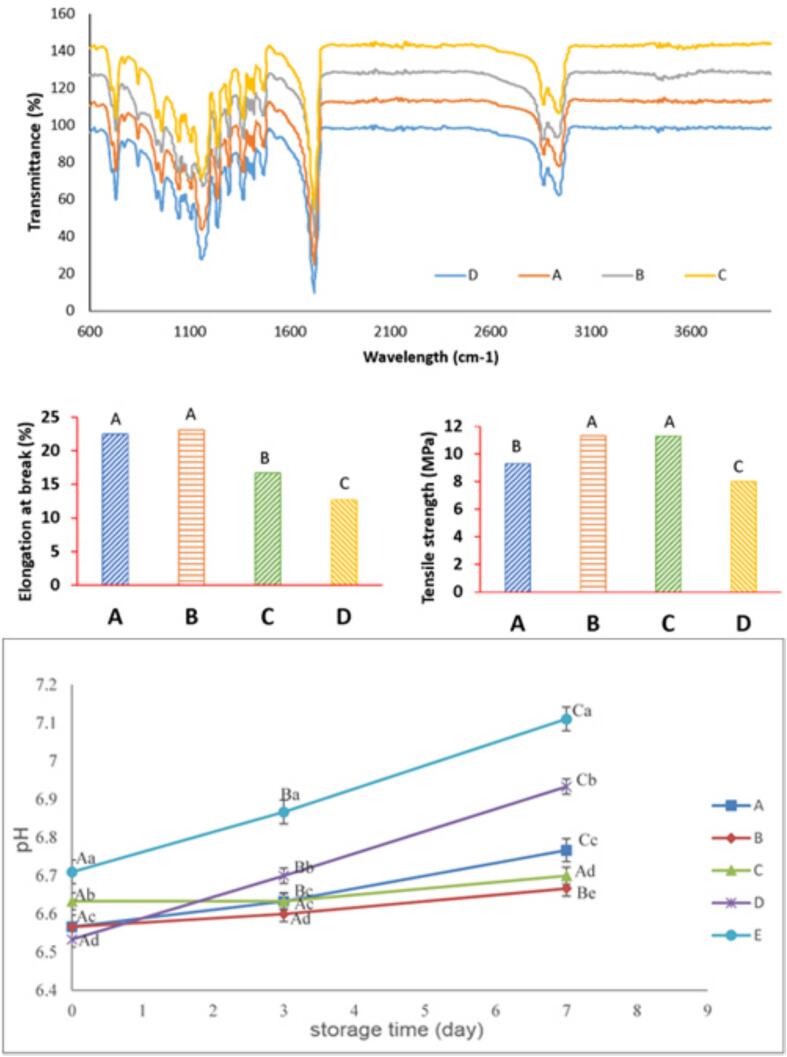
The FTIR spectra of the 1% AgNPs, 1% AgNPs +1% HCNs, 1% AgNPs +2% HCNs, and 1% HCNs are shown in Fig. 3. The FTIR spectrum of all groups included specific absorption peaks of –CH2 groups in the range of 2940 cm−1, and the peak that appeared in the range of 2864 cm−1 was due to the asymmetric CH2. From the interaction between the C O groups, peaks around 1718 cm−1, associated with the stretching absorption of the carbonyl group, were formed. In addition, the peak of the C-O-C spectrum was in the range of 1292 cm−1. The peak in the range of 1237 cm−1 indicated oxygen bridge tension and the range of 933 cm−1 showed C—H deformation.
Fig. 3.
Graphs for prepared films and pH values of meat samples during storage time containing A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs.
The mechanical properties of the fabricated films, including tensile strength (TS) and elongation at break (EB), are demonstrated in Fig. 3. According to the results of EB, there was no significant difference between Group A (containing 1% of AgNPs) and Group B (containing 1% of AgNPs +1% of HCNs) (P > 0.05). By increasing the HCN content to 2%, the EB reduced (group C) (P < 0.05). Group D, with just 1% of HCNs, showed significantly the lowest EB values. Regarding TS (Fig. 3), it can be concluded that the addition of HCNs increased TS from around 9.3% to 11.8% (P < 0.05). Increasing HCNs from 1% to 2% did not significantly affect TS (P > 0.05). The results also indicated that treated samples with 1% AgNPs had significantly higher TS than those treated with 1% HCNs. Furthermore, the combination of the AgNPs and HCNs showed the highest TS values compared to samples containing AgNPs or HCNs. NPs have been found to impact the mechanical properties of PCL-based films significantly. The observed variations in TS and EB among different film formulations underscore the intricate interplay between film composition and mechanical performance. Recent studies have highlighted the influence of nanoparticle dispersion and interfacial interactions within polymer matrices on the mechanical properties of nanocomposite films. The combination of AgNPs and HCNs in the films not only enhanced tensile strength but also contributed to improved toughness and elasticity, as evidenced by the increased elongation at break values observed in certain formulations (Chen, Ma, Hu, & Fei, 2021). Research has shown that adding NPs into the PCL-based films improved properties like tensile strength, elasticity, and toughness. This means the films become more resistant to breakage and less likely to deform under stress (Toncheva et al., 2018).
3.2.3. Atomic force microscopy (AFM)
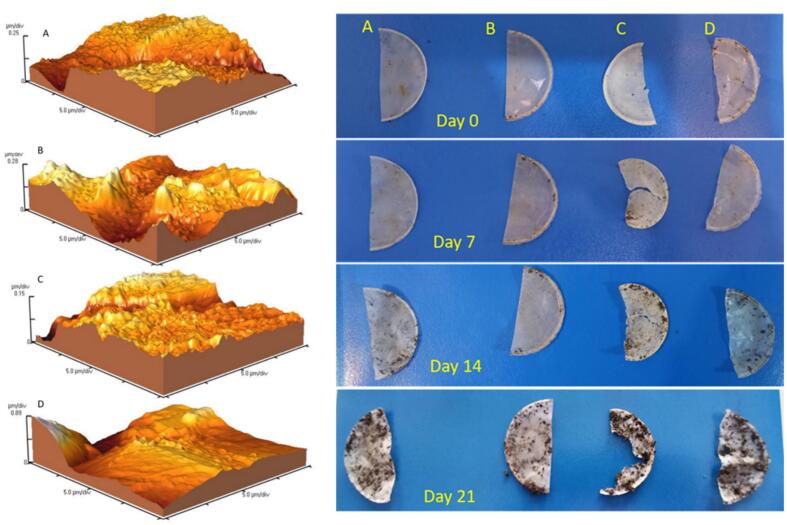
AFM was used to examine the topography and surface roughness of the samples (Kan et al., 2023). Based on our findings presented in Fig. 4., it is evident that the surfaces of all four coatings exhibited uniformity. This observation implies that the size distribution of the NPs within the layers remained consistent across all four coatings. However, unevenness was observed in some areas. In films containing silver NPs, the surface roughness parameter for nanofibers was approximately 170–80 nm, whereas this value was about 29 nm for group C (control). The results showed that the presence of AgNPs increased the surface roughness, and the presence of HCNs decreased the surface roughness. Subsequently, reduced surface roughness indicates a decreased contact area and, more importantly, a decreased migration rate of the NPs. The outcomes of the present study showed that at a constant concentration of AgNPs, adding HCNs to the films reduced the level of surface roughness.
Fig. 4.
AFM analysis to monitor the surface roughness of the films; and the degradability rate of prepared films using in-earth technique for films containing A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs.
3.2.4. Biodegradability
The investigation into the degradability of films sheds light on their environmental implications, particularly in the context of addressing plastic pollution concerns. As highlighted by the reviewer, the growing awareness of plastic pollution and its environmental impact underscores the importance of assessing the biodegradability of packaging materials. The results of the film's degradability showed that the films containing AgNPs did not produce a significant degradability rate, whereas in other groups containing HCNs, an effective degradability rate was observed suggesting a potential advantage in terms of environmental sustainability. This enhanced degradability presents an opportunity to mitigate the long-term accumulation of plastic waste in the environment, offering a promising alternative to conventional packaging materials (Fig. 4.). By observing the films containing HCNs and comparing them, the degradability rate changes in the different groups are quite evident. Group B showed a high degradability rate due to the increase in HCNs compared to group A. Group C had the highest degradability rate. According to the obtained results, it can be concluded that several factors increase the degradability rate, particularly the hydrophilicity and degradability of HCNs (Zhang, Ding, Povey, & York, 2008). Because HCNs are inherently degradable, their presence in the film structure increases the degradation rate. Additionally, the presence of AgNPs in the films of A and B reduced the amount of water penetration, leading to a delay in the water supply to HCNs and reducing the degradability rate. By comparing films A and C, the significant role of the HCNs in the destruction of the films was confirmed. By incorporating HCNs into film structures, it may be possible to accelerate the degradation process, thereby reducing the environmental footprint associated with packaging materials. Therefore, the biodegradability of HCN-containing films opens up avenues for sustainable waste management practices. Films that degrade over time can alleviate the burden on landfill sites and minimize the risk of environmental contamination, contributing to a more circular economy approach to packaging. The findings of this study underscore the potential of HCN-containing films to make significant contributions to sustainability in the packaging industry and offer a promising avenue for addressing concerns related to plastic pollution and advancing the development of sustainable packaging solutions that align with current environmental priorities.
3.2.5. Water vapor transmission rate (WVTR)
The permeability of the prepared films was measured based on the water vapor transfer rate. The film A, including 1% AgNPs, showed the highest permeability rate to water vapor (8.84 g.h−1. m−2). The results indicated that by increasing the concentration of HCNs, the permeability to water vapor decreased (P > 0.05). According to the results, WVTR decreased from 8.84 (g.h−1. m−2) in the film containing 1% AgNPs to 8.16 (g.h−1. m−2) in films containing 1% AgNPs +1% HCNs and to 2.72 (g.h−1. m−2) in the films containing 1% AgNPs and 2% HCNs, indicating the effects of HCNs in the reduction of WVTR. Shankar, Wang, & Rhim (2018) also indicated that the WVP of the PLA-based nanocomposite films decreased in the presence of ZnONPs.
3.2.6. pH values, Thiobarbituric acid rate (TBARs), Total volatile basic nitrogen (TVB-N), and Peroxide value (PV)
In this section, the pH values, TBARs, TVB-N, and PV have been analyzed to understand their impact on meat quality and shelf-life extension. According to Fig. 3, the pH of meat samples increased throughout the refrigerated period, which is due to the autolytic activity of the autochthonous enzymes, and the synthesis of alkaline biometabolits of psychrotrophic bacteria, and lactic acid bacteria. The increase in pH values, albeit less pronounced in coated samples, reflects the complex interplay between microbial activity, enzymatic processes, and the buffering capacity of the meat matrix. These coatings likely create a barrier that impedes the diffusion of alkaline biometabolites, thereby mitigating pH rise and preserving the sensory attributes of the meat (Yaghoubi et al., 2021). pH increase was more in control (p ≤ 0) in comparison to pH values in coated samples. Similar antibacterial attributes of treatments have been reported by Berizi, Hosseinzadeh, Shekarforoush, and Barbieri (2018)and Yaghoubi et al. (2021) in chicken meat and other food model systems.
TBARs value is one of the most critical indicators in meat and meat products for the measurement of oxidation secondary products (particularly aldehydes from polyunsaturated fatty acids). The results of TBARs values indicated that the TBAR values of all samples increased throughout the storage period (Fig. 5). According to the results, treated samples with 1% AgNPs +2% HCNs (0.651 mg MDA/kg) and control samples (1.08 mg MDA/kg) displayed the lowest and the highest TBAR values, respectively. The reduction in TBARs values in coated samples can be attributed to several mechanisms. The nanocomposite coatings act as a physical barrier, limiting the exposure of meat lipids to oxygen and thereby reducing lipid oxidation (Sogut & Seydim, 2019). Moreover, the high antioxidant attributes of treatments would also lead to a lesser increase in TBARS values throughout the keeping period. The presence of nanoparticles, such as AgNPs, may exert antioxidant effects by scavenging free radicals and chelating pro-oxidant metal ions. Additionally, the incorporation of natural compounds like HCNs may enhance the antioxidative properties of the coatings through their ability to donate hydrogen atoms or electrons, thereby interrupting lipid oxidation chain reactions. Hence, as expected, films containing AgNPs and HCNs allowed to extend the shelf life of Tilapia samples by their antioxidative attributes. Similar results were also reported by Majumder et al. (2023), who assessed the effects of AgNPs in soy protein isolate (SPI)-based films and reported high antioxidant attributes in packaged chicken breast meat. The authors indicated that 3.76% of AgNPs/SPI films scavenged ∼4.5% of DPPH in 48 h.
Fig. 5.
Chemical analysis and microbial count of meat samples during storage time. A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs, E: Control.
The peroxide value results showed that the oxidation increased throughout the storage period (Fig. 3). After three days, the amount of peroxide value in the samples containing 1% HCNs was more significant than that in the other groups. In addition, samples containing AgNPs had a higher amount of peroxide value than group B (1% AgNPs +1% HCNs) and C (1% AgNPs and 2% HCNs). These changes in the amount of peroxide in the groups became more significant by increasing the storage period. The progressive increase in PV values, although attenuated in coated samples, highlights the ongoing oxidative processes occurring during storage. Despite the antioxidative properties of the coatings, oxidation reactions inevitably occur, albeit at a slower rate, due to the presence of residual oxygen and pro-oxidant factors.
TVB-N is an important indicator for assessing the quality and shelf-life of meat and meat products (Yaghoubi et al., 2021), and its increase is closely associated with the degradation of protein or non-protein nitrogenous compounds by spoilage bacteria and enzymes (Li, Zhu, & Lin, 2011). The TVB-N results of fish samples during storage at 4 °C are represented in Fig. 5. Among keeping time. The TVB-N values in all fish samples increased exponentially (p ≤ 0.05) higher in control (18.61 vs. 4.46 mg/100 g for control and packed samples with films containing 1% AgNPs +2% HCNs, respectively). Packed fish samples with HCNs and AgNPs inhibited synthesis of volatile nitrogen bases until day 7 (5.93, 4.76, 4.46, and 6.26 mg/100 g for samples treated with films containing 1% AgNPs, 1% AgNPs +1% HCNs, 1% AgNPs +2% HCNs, and 1% HCNs, respectively), which is similar to report of Alirezalu et al. (2021) who showed a reduced TVB-N formation in samples packed by polyamide-alginate incorporated with NPs of natural preservatives on meat products. The low TVB-N values corresponded with inhibited microbial growth and pH decrease. The inhibition of TVB-N formation in coated samples suggests effective control of microbial spoilage and enzymatic degradation of nitrogenous compounds. The nanocomposite coatings likely create a hostile environment for microbial growth by releasing antimicrobial agents or modifying the surface properties of the meat, thereby impeding the activity of spoilage bacteria and enzymes. Furthermore, the presence of nanoparticles may disrupt the cellular membranes of microorganisms, leading to cell lysis and inhibition of metabolic processes involved in nitrogen compound degradation (Ayaseh et al., 2022; Peighambardoust et al., 2022; Yaghoubi et al., 2021). Overall, the observed changes in pH values, TBARs, TVB-N, and PV highlight the efficacy of the coatings in preserving meat quality and extending shelf-life by mitigating oxidation and inhibiting microbial growth. Therefore, the integration of nanocomposite coatings represents a promising approach for improving the preservation of meat quality and extending shelf-life which may lead to developing more effective strategies to meet the growing demand for safe and high-quality meat products in the food industry.
3.2.7. Microbial activity
On the first day, the total thermophilic bacteria count was 2.31–2.39 Log CFU/g (less than the control 3.27 Log CFU/g) (Fig. 5). The bacterial count for psychrotrophic bacteria was 2.83 and 3.17 Log CFU/g, compared with the control (3.35 Log CFU/g). These bacterial counts indicated high antimicrobial attributes of films containing AgNPs and HCNs in fish samples. Films containing 1% AgNPs +2% HCNs led to approximately 2.1 Log CFU/g and 2.23 Log CFU/g reduction in total thermophilic and psychrotrophic bacteria, respectively, from those obtained by control, which is similar to those reported by Alirezalu et al. (2021) on chicken meat packed in polyamide-alginate casing + NPs of natural preservatives. Films containing 1% AgNPs +2% HCNs have an impact on impact on fish meat quality and shelf-life. Decrease the oxygen transfer of the films and antimicrobial properties cause extended shelf life. This study showed that the simultaneous presence of Ag and HCNs in the film can control the growth of microorganisms. However, the presence of individual NPs slightly affects the development of microorganisms. According to Fig. 5, coliforms increased throughout the keeping period. The rate of this increase was exponentially higher in control meat (5.75 Log CFU/g) compared to values of 2.55 Log CFU/g observed in packed meat samples in films containing 1% AgNPs +2% HCNs. The count of Staphylococcus aureus bacteria also followed a similar trend over time. The packed samples with films containing 1% AgNPs +2% HCNs and control meat displayed the lowest and highest increase among refrigerated periods, respectively.
The incorporation of 1% AgNPs +2% HCNs, caused antimicrobial properties against total thermophilic and psychrotrophic bacteria, coliforms, and Staphylococcus aureus. The mechanism underlying the antimicrobial activity of these films can be attributed to multiple factors. The incorporation of 1% AgNPs +2% HCNs reduces the oxygen transfer rate, creating an environment less conducive to microbial growth. Additionally, the release of Ag NPs from the films may interact with microbial cells, disrupting cellular functions and inhibiting their proliferation. This dual mode of action, combining reduced oxygen availability with the antimicrobial properties of Ag NPs, synergistically contributes to the observed reductions in bacterial counts. Furthermore, Tong et al. (2023) similarly reported the antimicrobial properties of SA films incorporated with cinnamic acid. Majumder et al. (2023) also mentioned the inhibition impact of soy protein isolate films containing AgNPs on microbial growth in chicken breast meat. 3.76% AgNP/SPI reduced (p ≤ 0) a 2.62 log10 of S. aureus and 2.14 log10 of S. typhimurium.
3.2.8. Evaluation of NPs migration into Tilapia tissue
According to the results during refrigerated storage, the NPs in Tilapia tissue were observed to increase over time (Table 1). Initially, the NPs migration rate was measured to be under 0.001 ICP-MS mg/l on the first day of storage, gradually escalating to under 0.02 ICP-MS mg/l at day 3 and 0.05 ICP-MS mg/l at day 7 for group A, and under 0.01 ICP-MS mg/l at day 3 and under 0.02 ICP-MS mg/l at day 7 for both groups B and C. These findings suggest a progressive increase in NP migration into the Tilapia tissue during the storage period. However, it is noteworthy that the presence of AgNPs and HCNs in films appeared to decrease the migration of NPs compared to the presence of AgNPs alone. This observation underscores the potential role of film composition in mitigating NP migration into food matrices. Nanoparticle migration raises concerns due to their potential bioaccumulation and ingestion by consumers, which could lead to unintended health consequences such as cellular toxicity and genotoxicity. Stu+dies have also highlighted the need for comprehensive risk assessment and regulatory frameworks to ensure the safety of NPs-containing food packaging materials. Strategies to mitigate potential risks include encapsulating nanoparticles within food packaging materials, utilizing alternative antimicrobial agents, and implementing rigorous testing protocols. Future research should focus on evaluating the long-term effects of nanoparticle exposure on both food safety and human health, as well as exploring mechanisms of nanoparticle interaction with biological systems to address knowledge gaps in nanoparticle safety and food packaging technology (Duncan, 2011; Johnston et al., 2010). (See Table 2.)
Table 1.
WVTR analysis of the prepared films.
| Groups | WVTR (g.h−1. m−2) |
ICP-MS mg/l (ppm) |
||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | ||
| A | 8.84 | < 0.001 | < 0.02 | <0.05 |
| B | 8.16 | < 0.001 | < 0.01 | < 0.02 |
| C | 2.72 | < 0.001 | < 0.01 | < 0.02 |
| D | 5.89 |
A: 1% AgNPs, B: 1% AgNPs and 1% HCNs, C: 1% AgNPs and 2% HCNs, D: 1% HCNs.
Table 2.
Evaluation of the NPs migration into Tilapia tissue.
| Groups |
ICP-MS mg/l (ppm) |
||
|---|---|---|---|
| Day 0 | Day 3 | Day 7 | |
| A | < 0.001 | < 0.02 | <0.05 |
| B | < 0.001 | < 0.01 | < 0.02 |
| C | < 0.001 | < 0.01 | < 0.02 |
A: 1% Ag NPs, B: 1% Ag NPs and 1% HCNs, C: 1% Ag NPs and 2% HCNs.
4. Conclusion and future perspective
In this study, we successfully developed SA films incorporating AgNPs and HCNs, which demonstrated multifunctional properties ideal for Tilapia preservation. These films exhibited remarkable antioxidant and antimicrobial activities while maintaining biodegradability and mechanical integrity, making them suitable for food packaging applications. Specifically, samples packed in films containing 1% AgNPs +2% HCNs showed superior performance, displaying lower levels of TBARs, PV, pH, and TVN compared to the control group throughout the storage period. Our findings signify significant progress in the development of innovative packaging solutions aimed at extending the shelf-life of meat and meat products. By leveraging the synergistic effects of AgNPs and HCNs, we not only achieved enhanced preservation efficacy but also contributed to the production of safer and environmentally friendly packaging materials. The observed inhibitory effects against spoilage and food-borne microorganisms highlight the potential of these active films to address food safety concerns and meet consumer demand for healthier and sustainable packaging options. Moving forward, future research should focus on optimizing film formulations tailored to specific food packaging applications. This includes exploring different combinations of antimicrobial agents and biopolymers to enhance film performance and meet the requirements of diverse food matrices. Additionally, efforts should be directed toward addressing safety concerns associated with nanoparticle migration from packaging materials into food products. Comprehensive risk assessments and migration studies are essential for ensuring the safety and regulatory compliance of nanocomposite packaging materials.
In summary, the development of SA films containing AgNPs and HCNs represents a promising approach for improving the quality and shelf-life of fish meat and other perishable food items. By providing insights into the practical implications of our findings and identifying future research directions, we aim to contribute to the advancement of sustainable packaging technologies and the promotion of food safety and quality in the food industry.
Funding declaration
This research received no specific funding.
Data availibility statement.
The data that support the findings of this study are available upon the request.
CRediT authorship contribution statement
Negin Ahmadi: Writing – review & editing, Writing – original draft, Validation, Software, Project administration, Methodology, Formal analysis. Hamed Ahari: Supervision, Project administration, Methodology. Amirali Anvar: Supervision. Kianoush Khosravi-Darani: Writing – review & editing, Supervision. Maryam Gharachorloo: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study received no financial support
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101490.
Appendix A. Supplementary data
Supplementary material.
Data availability
Data will be made available on request.
References
- Afrin F., Islam M., Rasul M., Sarkar M., Yuan C., Shah A. Effects of seaweed extracts on the quality and shelf life of Nile tilapia (Oreochromis niloticus) fillets during frozen storage. Food Chemistry Advances. 2023;3 [Google Scholar]
- Alirezalu K., Hesari J., Yaghoubi M., Khaneghah A.M., Alirezalu A., Pateiro M., Lorenzo J.M. Combined effects of ε-polylysine and ε-polylysine nanoparticles with plant extracts on the shelf life and quality characteristics of nitrite-free frankfurter-type sausages. Meat Science. 2021;172 doi: 10.1016/j.meatsci.2020.108318. [DOI] [PubMed] [Google Scholar]
- Attaran S.A., Hassan A., Wahit M.U. Materials for food packaging applications based on bio-based polymer nanocomposites: A review. Journal of Thermoplastic Composite Materials. 2017;30(2):143–173. [Google Scholar]
- Ayaseh A., Alirezalu K., Yaghoubi M., Razmjouei Z., Jafarzadeh S., Marszałek K., Khaneghah A.M. Production of nitrite-free frankfurter-type sausages by combining ε-polylysine with beetroot extracts: An assessment of microbial, physicochemical, and sensory properties. Food Bioscience. 2022;49 [Google Scholar]
- Balakrishnan P., Geethamma V., Gopi S., Thomas M.G., Kunaver M., Huskić M.…Thomas S. Thermal, biodegradation and theoretical perspectives on nanoscale confinement in starch/cellulose nanocomposite modified via green crosslinker. International Journal of Biological Macromolecules. 2019;134:781–790. doi: 10.1016/j.ijbiomac.2019.05.088. [DOI] [PubMed] [Google Scholar]
- Berizi E., Hosseinzadeh S., Shekarforoush S.S., Barbieri G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. International Journal of Biological Macromolecules. 2018;106:1004–1013. doi: 10.1016/j.ijbiomac.2017.08.099. [DOI] [PubMed] [Google Scholar]
- Carbone M., Donia D.T., Sabbatella G., Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. Journal of King Saud University-Science. 2016;28(4):273–279. [Google Scholar]
- Chen Q., Ma J., Hu Y., Fei P. Multifunctional antibacterial films with silver nanoparticles reduced in situ by lemon juice. Food Chemistry. 2021;365:130517. doi: 10.1016/j.foodchem.2021.130517. [DOI] [PubMed] [Google Scholar]
- Dilbaghi N., Kaur H., Ahuja M., Arora P., Kumar S. Synthesis and evaluation of ciprofloxacin-loaded carboxymethyl tamarind kernel polysaccharide nanoparticles. Journal of Experimental Nanoscience. 2014;9(10):1015–1025. [Google Scholar]
- Duncan T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. Journal of Colloid and Interface Science. 2011;363(1):1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili J., Pirzadeh K., Pakrooyan M., Lukolayeh M.E., Kırboğa K.K. Synthesis of cellulose nanocrystals from spinach waste for insulin delivery: Comparison to chitosan nanoparticles. New Journal of Chemistry. 2024;48:7953–7963. [Google Scholar]
- Johnston H.J., Hutchison G., Christensen F.M., Peters S., Hankin S., Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Critical Reviews in Toxicology. 2010;40(4):328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- Kan G., Zi Y., Li L., Gong H., Peng J., Wang X., Zhong J. Curcumin-encapsulated hydrophilic gelatin nanoparticle to stabilize fish oil-loaded Pickering emulsion. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2023.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavuncuoglu H., Yalcin H., Dogan M. Development of (TiO2-ZnO)/LDPE based active nanocomposite films and detection of migration to minced beef during storage using response surface methodology. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134278. [DOI] [PubMed] [Google Scholar]
- Khursheed S., Dutta J., Ahmad I., Rather M.A., Badroo I.A., Bhat T.A.…Qadri T. Biogenic silver nanoparticles: Synthesis, applications and challenges in food sector with special emphasis on aquaculture. Food Chemistry. 2023;X doi: 10.1016/j.fochx.2023.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshani R., Eiyegbenin J.E., Wang Y., van de Ven T.G. Synthesis and characterization of hairy aminated nanocrystalline cellulose. Journal of Colloid and Interface Science. 2022;607:134–144. doi: 10.1016/j.jcis.2021.08.172. [DOI] [PubMed] [Google Scholar]
- Koshani R., Zhang J., van de Ven T.G., Lu X., Wang Y. Modified hairy nanocrystalline cellulose as photobactericidal nanofillers for food packaging application. ACS Sustainable Chemistry & Engineering. 2021;9(31):10513–10523. [Google Scholar]
- Li M., Zhu L., Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environmental Science & Technology. 2011;45(5):1977–1983. doi: 10.1021/es102624t. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang X., Wu Y., Hou J., Zhang S., Zhou N., Wang X. Toxicity responses of different organs of zebrafish (Danio rerio) to silver nanoparticles with different particle sizes and surface coatings. Environmental Pollution. 2019;246:414–422. doi: 10.1016/j.envpol.2018.12.034. [DOI] [PubMed] [Google Scholar]
- Ly B., Thielemans W., Dufresne A., Chaussy D., Belgacem M. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Composites Science and Technology. 2008;68(15–16):3193–3201. [Google Scholar]
- Mahrous F., Koshani R., Tavakolian M., Conley K., van de Ven T.G. Formation of hairy cellulose nanocrystals by cryogrinding. Cellulose. 2021;28:8387–8403. [Google Scholar]
- Majumder S., Huang S., Zhou J., Wang Y., George S. Tannic acid-loaded halloysite clay grafted with silver nanoparticles enhanced the mechanical and antimicrobial properties of soy protein isolate films for food-packaging applications. Food Packaging and Shelf Life. 2023;39 [Google Scholar]
- Morantes D., Muñoz E., Kam D., Shoseyov O. Highly charged cellulose nanocrystals applied as a water treatment flocculant. Nanomaterials. 2019;9(2):272. doi: 10.3390/nano9020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhametov A., Shamekova M., Dautkanova D., Kazhymurat A., Ilyassova G. Journal of agriculture and food research. Journal of Agriculture and Food Research. 2023;11 [Google Scholar]
- Oney-Montalvo J.E., Dzib-Cauich D.A., de Jesús Ramírez-Rivera E., Cabal-Prieto A., Can-Herrera L.A. Applications of polycaprolactone in the food industry: A review. Czech Journal of Food Sciences. 2024;42(2):77–84. [Google Scholar]
- Onyeaka H., Passaretti P., Miri T., Al-Sharify Z.T. The safety of nanomaterials in food production and packaging. Current Research in Food Science. 2022;5:763–774. doi: 10.1016/j.crfs.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peighambardoust S.H., Yaghoubi M., Hosseinpour A., Alirezalu K., Soltanzadeh M., Dadpour M. Development and application of dual-sensors label in combination with active chitosan-based coating incorporating yarrow essential oil for freshness monitoring and shelf-life extension of chicken fillet. Foods. 2022;11(21):3533. doi: 10.3390/foods11213533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A., Razavi S.M.A., Shahrampour D. Fabrication and characterization of biodegradable active films with modified morphology based on polycaprolactone-polylactic acid-green tea extract. International Journal of Biological Macromolecules. 2022;205:341–356. doi: 10.1016/j.ijbiomac.2022.02.070. [DOI] [PubMed] [Google Scholar]
- Shankar S., Wang L.F., Rhim J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Materials Science and Engineering: C. 2018;93:289–298. doi: 10.1016/j.msec.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Sogut E., Seydim A.C. The effects of chitosan-and polycaprolactone-based bilayer films incorporated with grape seed extract and nanocellulose on the quality of chicken breast fillets. Lwt. 2019;101:799–805. [Google Scholar]
- Tavakolian M., Lerner J., Tovar F.M., Frances J., van de Ven T.G., Kakkar A. Dendrimer directed assembly of dicarboxylated hairy nanocellulose. Journal of Colloid and Interface Science. 2019;541:444–453. doi: 10.1016/j.jcis.2019.01.100. [DOI] [PubMed] [Google Scholar]
- Toncheva A., Khelifa F., Paint Y., Voué M., Lambert P., Dubois P., Raquez J.-M. Fast IR-actuated shape-memory polymers using in situ silver nanoparticle-grafted cellulose nanocrystals. ACS Applied Materials & Interfaces. 2018;10(35):29933–29942. doi: 10.1021/acsami.8b10159. [DOI] [PubMed] [Google Scholar]
- Tong W.Y., Rafiee A.R.A., Leong C.R., Tan W.-N., Dailin D.J., Almarhoon Z.M.…Chuah L.F. Development of sodium alginate-pectin biodegradable active food packaging film containing cinnamic acid. Chemosphere. 2023;336 doi: 10.1016/j.chemosphere.2023.139212. [DOI] [PubMed] [Google Scholar]
- Van De Ven T.G., Sheikhi A. Hairy cellulose nanocrystalloids: A novel class of nanocellulose. Nanoscale. 2016;8(33):15101–15114. doi: 10.1039/c6nr01570k. [DOI] [PubMed] [Google Scholar]
- Yaghoubi M., Ayaseh A., Alirezalu K., Nemati Z., Pateiro M., Lorenzo J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers. 2021;13(5):716. doi: 10.3390/polym13050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Fei X., He Y., Li H. Poly (lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. International Journal of Biological Macromolecules. 2021;186:770–779. doi: 10.1016/j.ijbiomac.2021.07.097. [DOI] [PubMed] [Google Scholar]
- Zeng A., Yang R., Tong Y., Zhao W. Functional bacterial cellulose nanofibrils with silver nanoparticles and its antibacterial application. International Journal of Biological Macromolecules. 2023;235 doi: 10.1016/j.ijbiomac.2023.123739. [DOI] [PubMed] [Google Scholar]
- Zhai X., Zhou S., Zhang R., Wang W., Hou H. Antimicrobial starch/poly (butylene adipate-co-terephthalate) nanocomposite films loaded with a combination of silver and zinc oxide nanoparticles for food packaging. International Journal of Biological Macromolecules. 2022;206:298–305. doi: 10.1016/j.ijbiomac.2022.02.158. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ding Y., Povey M., York D. ZnO nanofluids–A potential antibacterial agent. Progress in Natural Science. 2008;18(8):939–944. [Google Scholar]
- Zhang M., Yang B., Yuan Z., Sheng Q., Jin C., Qi J., Yu M., Liu Y., Xiong G. Preparation and performance testing of corn starch/pullulan/gallic acid multicomponent composite films for active food packaging. Food Chemistry: X. 2023;19 doi: 10.1016/j.fochx.2023.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.