Abstract
Although germ cell tumours can appear in childhood, they are most common around the age of 30. These tumours are highly responsive to chemotherapy, and even cases of relapse have relatively high cure rates. There is limited literature on patients diagnosed after the age of 50, and no specific trials have been carried out in this context. These patients, considered 'elderly', are treated with the same cisplatin-containing combinations as younger patients, despite the higher toxicity. This report presents an observation of a giant testicular mixed germ cell tumour discovered in a 75-year-old patient.
Keywords: Testicular cancer, Mixed germ cell tumours, Scrotum swelling, Elderly patients
1. Introduction
Testicular tumours account for 1–3% of male cancers.1 Over 90 % of these cancers develop in germ cells, resulting in two main types of testicular germ tumour: seminomas and non-seminomas. The latter comprises embryonal carcinomas, teratomas, choriocarcinomas, and yolk sac tumours, this tumour type is known to be more aggressive and is commonly found in individuals aged between 15 and 34.2 3 Early diagnosis is aided by the initial clinical sign of a large bursa that gradually progresses.4 The current cure rate is high despite the aggressive nature of the disease.5 Treatment typically involves surgical resection combined with adjuvant chemotherapy and/or radiotherapy. It is important to note that this tumour type is exceedingly rare before puberty,6 and its frequency decreases after the age of 50..7
2. Case presentation
We report here the case of a 75-year-old patient, with a history of smoking and chronic cannabis use, who was admitted to our institution for medical management due to an increase in the size of his right bursa, described as "chronic large bursa". This case is of particular interest because of its unusual presentation and the clinical challenges encountered in its management.
The patient was confronted with the evolution of his condition for 5 months before consulting, mainly due to the fear and embarrassment associated with his medical situation. The testicular mass, discovered by self-palpation, had progressively increased in size to become swollen, hard, and accompanied by an incapacitating sensation of testicular heaviness. This development was accompanied by a deterioration in general condition.
On physical examination, the patient presented with a large swelling of the right bursa, hard to the touch and pushing back the contralateral testicle(Fig. 1). A scrotal ultrasound revealed a large tissue process at the epicenter of the right bursa, measuring 17 × 16 cm and compressing the contralateral testis (Fig. 2). Suspicious adenopathies were detected in the bilateral inguinal and inter-aortic-caval areas.
Fig. 1.
Aspect of the scrotum on clinical examination showing a very enlarged bursa.
Fig. 2.
Sonographic appearance of the testicular mass (A).
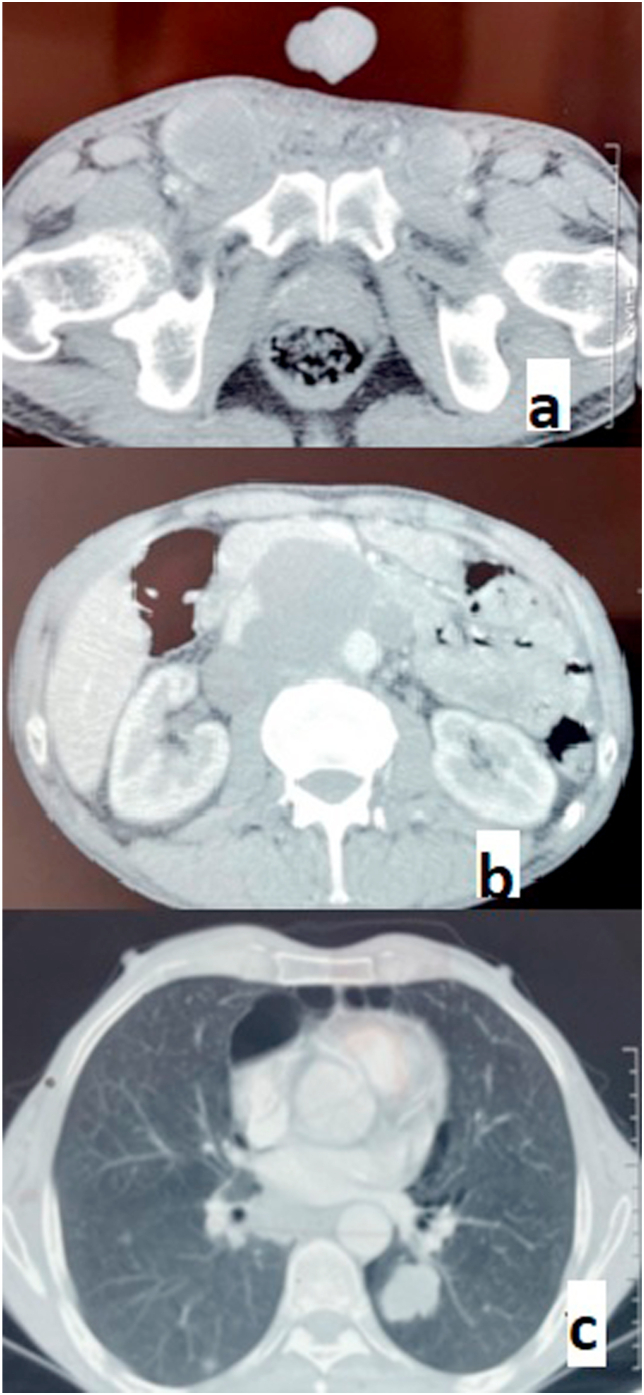
Tumour markers were significantly elevated, with alpha-fetoprotein (AFP) reaching 23,640 ng/ml and lactate dehydrogenase (LDH) at 807 IU/l. However, total human chorionic gonadotropin hormone (HCG) levels were normal. A thoracic-abdominal-pelvic CT scan confirmed the presence of para-aortic lymph nodes and lung metastases.(Fig. 3).
Fig. 3.
Images of CT sections showing: inguinal (a) and inter-aortic-caval (b) lymph node metastases and lung metastases (b).
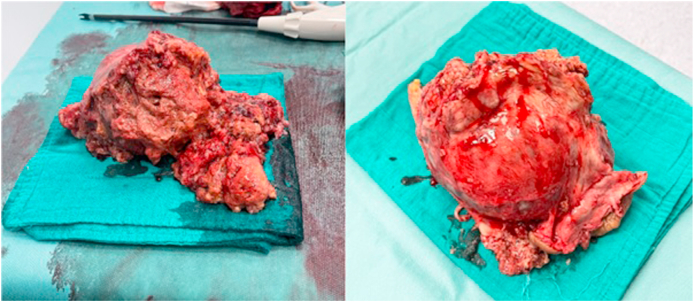
After discussion at a multidisciplinary consultation meeting (RCP), the patient underwent emergency radical right orchiectomy via a double inguinal and scrotal route, due to the large size of the tumour. A right inguinal incision was made to locate, isolate and clamp the spermatic cord. A second incision was made on the median raphe to dissect the tumour, which was impressively large and appeared to invade the scrotum. The tumour was excised in its entirety, leaving an island of adherent skin on its surface (Fig. 4).
Fig. 4.
Macroscopic appearance of the surgical specimen.
Pathological examination of the surgical specimen confirmed the presence of a mixed germ cell tumour, combining a yolk sac tumour with an embryonal carcinoma, with infiltration of the albuginea and vagina, and contact with the scrotal skin without ulceration. Vascular emboli were observed in the section of spermatic cord. The tumour was classified as stage IIIc (pT3N3M1a), measuring 3 kg 603 g, with dimensions of 32 cm × 21 cm.
The patient was then referred to the oncology department and a new tumour marker assessment was performed, which showed an AFP level of 10,478 ng/ml and an LDH level of 627 UI/L. The testicular tumour was classified as poor prognosis according to the IGCCCG classification and the patient received four cycles of BEP-based chemotherapy (cisplatin, etoposide and bleomycin). After the first course of chemotherapy, serum marker levels decreased significantly, with LDH at 217 UI/L and AFP at 429 ng/ml. After completion of chemotherapy, AFP normalised with significant clinical improvement and complete radiological regression of metastases and retroperitoneal lymphadenopathy. Retroperitoneal lymph node dissection was therefore not required. The patient was subsequently followed up clinically, biologically (markers) and radiologically (thoraco-abdomino-pelvic imaging) every three months. After 12 months of follow-up, the physical examination, computed tomography scan and markers (AFP of 8 UI/ml and LDH of 179 UI/L) were normal, indicating a complete response to treatment.
3. Discussion
Mixed germ cell tumours are extremely rare in both prepubertal and elderly patients, and their incidence and clinicopathological features are poorly characterised in the medical literature. Pugh8 documented 153 cases of testicular cancer in men over 60 years of age, with lymphoma constituting the majority (70 cases, 46 %) of primary testicular tumours in this population. Germ cell tumours, including spermatocytic, cord and stromal tumours, accounted for the remaining cases (44 cases, 29 %), alongside a heterogeneous mix of sarcomas and other malignancies (29 cases, 19 %). Elevated serum marker levels often reflect the different cell types present in the primary testicular tumour and/or metastatic lesions.9 Conversely, malignant germ cell tumours (GCTs) in men aged 50 years and older have different characteristics from their younger counterparts. They are particularly rare in this age group, with a sharp decline in incidence after the age of 65, a pattern that differs from the more common occurrence of GCTs in younger individuals.
The retrospective study by Fawaz Almutairi et al.10 provides valuable insights into malignant germ cell tumours (GCTs) in men aged 50 years and older, a population often overlooked in medical research. Covering the period from 2005 to 2021, the study shows a decline in GCT incidence after the age of 65, shedding light on age-specific patterns of tumour occurrence. Histologically, these tumours fall predominantly into two categories: seminoma and non-seminoma/mixed GCT, with seminoma accounting for 55 % of cases and non-seminoma/mixed GCT 32 %, particularly in men aged 50 and over.11 Mixed GCTs, when present, often include embryonal carcinoma, seminoma and yolk sac tumour, with embryonal carcinoma being the most common component at 77 %.10 12 In addition, a significant proportion of patients in this age group present with aggressive pathological features such as lymphovascular invasion, retroperitoneal/lymph node involvement and higher stage, accounting for 77 % of cases. Clinical follow-up data show that 14 % of patients aged 50 years and older die of disease-related causes.10
When compared to their younger counterparts, malignant GCTs in men aged 50 years and older tend to be larger, higher stage, more frequent vascular and rete testis invasion, and less associated with intratubular germ cell neoplasia, which is present in only 47 % of cases.10 Here, testicular cancer was diagnosed with a size of 17 × 16 cm. Lack of knowledge and awareness unnecessarily delays consultation at an early stage. It has been reported that men have little knowledge about their risk of testicular cancer, the early signs and how to carry out a self-examination.13 A Japanese study of 42 giant testicular tumours concluded that delayed consultation due to patient embarrassment was the main reason for tumour growth.14
Older patients derive similar benefits from chemotherapy as younger patients in the treatment of germ cell tumours, but challenges arise due to difficulty in tolerating regimens and increased risk of adverse outcomes. The primary chemotherapeutic agents used, namely bleomycin, cisplatin and etoposide, carry a higher risk of toxicity in the elderly. Bleomycin, which is excreted by the kidneys, is particularly problematic due to the age-related decline in glomerular filtration rate, which contributes to bleomycin-associated toxicities such as pneumonitis and reduced lung function. As a result, NCCN guidelines advise against bleomycin in patients over 50 with impaired renal or pulmonary function, and suggest carboplatin as an alternative to cisplatin in patients with renal impairment.15 A retrospective review found treatment complications leading to regimen changes, discontinuation or significant delays in 60 % of patients over 50,16 with 44 % experiencing febrile neutropenia. Prophylactic granulocyte-stimulating factors are recommended for all patients over 50 years of age. Despite increased toxicity, chemotherapy yields better outcomes in GCT patients over 50, warranting completion of treatment with curative intent and escalated supportive measures, including prophylactic granulocyte-stimulating factors, to effectively manage toxicity.16 Our patient successfully completed four cycles of BEP-based chemotherapy (consisting of cisplatin, etoposide and bleomycin) without any complications requiring discontinuation.
In the age group 50 years and older, the 5-year relative survival rate for germ cell tumours is significantly lower than in younger cohorts. Surveillance, Epidemiology, and End Result (SEER) data indicate a relative survival rate of 94 % for seminoma patients and 71 % for non-seminoma patients in this age category.17 Multiple factors contribute to this disparity, such as delayed diagnosis leading to less favourable disease staging, treatment intolerance, comorbidities, and suboptimal treatment approaches. Even after adjustment for stage and histology, studies based on SEER data show that diagnosis of germ cell tumours after age 50 is associated with worse survival.18 The leading cause of death is the tumour itself, accounting for 61 % of cases, followed by secondary non-GCT malignancies in 13 % of cases, with other non-cancer causes accounting for the remaining 26 %.18
4. Conclusion
It is highly unusual for patients to allow a tumour to grow to such a large size in developed countries, which benefit from extensive education and easy access to information. Fear, shame and embarrassment are certainly the most likely reasons why this patient delayed presenting. Additionally, these tumours in older men display a propensity for higher stage at presentation, more adverse pathologic features, and a more aggressive clinical course, highlighting the unique characteristics of GCTs in this demographic.
Informed consent
The patients consent was required, voluntary and informed.
CRediT authorship contribution statement
Reda Tariqi: Writing – original draft, Resources, Methodology, Funding acquisition, Data curation. Adam El Aboudi: Data curation. Abdelmounim Boughaleb: Investigation. Imad Boualaoui: Resources. Ahmed Ibrahimi: Project administration. Hachem El Sayegh: Supervision. Yassine Nouini: Validation.
Declaration of competing interest
The authors declare no competing interests.
References
- 1.Motzer Robert J., Jonasch Eric, Agarwal Neeraj, et al. Testicular cancer, Version 2.2015. J Natl Compr Cancer Netw. 2015 Jun;13(6):772–799. doi: 10.6004/jnccn.2015.0092. [DOI] [PubMed] [Google Scholar]
- 2.anagho E.A., McAninch J.W. In: Smith's General Urology. Tanagho E.A., McAninch J.W., editors. McGraw-Hill; New York: 2000. Genital tumors; pp. 422–428. [Google Scholar]
- 3.Ruf C., Isbarn H., Wagner W., Fisch M., Matthies C., et al. Changes in epidemiologic features of testicular germ cell cancer: age at diagnosis and relative frequency of seminoma are constantly and significantly increasing. Urol Oncol: Semin Original Investigat. 2014;32 doi: 10.1016/j.urolonc.2012.12.002. 33. e1–.e6. [DOI] [PubMed] [Google Scholar]
- 4.Minsal chile. Mdsd Clinical guidelines for testicular cancer in people aged 15 years and over (Guía clínica cáncer de testiculo en personas de 15 años y más) Minsal. 2010:6–10. [Google Scholar]
- 5.Valderrama-Gómez R.A., Condori-Saldaña J., Claros-Gutiérrez P.G., Claros-Matienzo C.A. Clinical CASE: testicular CANCER WITH METASTASES. Rev Méd-Cient “Luz Vida. 2011;2(1):76–80. [Google Scholar]
- 6.Pinkerton C.R. Malignant germ cell tumors in childhood. Eur J Cancer. 1997;33:895–901. doi: 10.1016/S0959-8049(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 7.AIRTUM/AIOM. 2021. https://www.aiom.it Available at:
- 8.Pugh R.C.B. In: Pathology of the Testis. first ed. Pugh R.C.B., editor. Blackwell Scientific Publications; Oxford: 1976. Testicular tumours-Introduction; pp. 139–163. [Google Scholar]
- 9.Morgan Tourne, Radulescu Camelia. Yves Allory Testicular germ cell tumors: histopathological and molecular features. Bull Cancer. April 2019;106(4):328–341. doi: 10.1016/j.bulcan.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Almutairi Fawaz, Geisler Daniel, Rammal Rayan, et al. Malignant germ cell tumors in men aged 50 years and over are associated with adverse pathologic features and higher stage at presentation. Ann Diagn Pathol. 2023;62 doi: 10.1016/j.anndiagpath.2022.152070. [DOI] [PubMed] [Google Scholar]
- 11.Berney D.M., Warren A.Y., Verma M., et al. Malignant germ cell tumours in the elderly: a histopathological review of 50 cases in men aged 60 years or over. Mod Pathol. 2008;21(1):54–59. doi: 10.1038/modpathol.3800978. [DOI] [PubMed] [Google Scholar]
- 12.Krag J.G., Barlebo H., Olsen J., et al. Testicular germ cell tumours in Denmark 1976–1980. Pathology of 1058 consecutive cases. Acta radiolOncologia. 1984;23:239–247. doi: 10.3109/02841868409136019. [DOI] [PubMed] [Google Scholar]
- 13.Ugboma H.A., Aburoma H.L. Public awareness of testicular cancer and testicular self-examination in academic environments: a lost opportunity. Clinics. 2011;66(7):1125–1128. doi: 10.1590/S1807-59322011000700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taisei K.I.N., Kitsukawa S., Shishido T., et al. Two cases of giant testicular tumor with widespread extension to the spermatic cord : usefulness of upfront chemotherapy. Hinyokika Kiyo -Acta Urologica Japonica. 1999 Mar;45(3):191–194. [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network Testicular cancer (Version 2.2022) https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf
- 16.Feldman D.R., Voss M.H., Jacobsen E.P., et al. Clinical features, presentation, and tolerance of platinum-based chemotherapy in germ cell tumor patients 50 years of age and older. Cancer. 2013;119(14):2574–2581. doi: 10.1002/cncr.28025. [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven R.H.A., Gondos A., Janssen-Heijnen M.L.G., et al. Testicular cancer in Europe and the USA: survival still rising among older patients. Ann Oncol. 2013;24(2):508–513. doi: 10.1093/annonc/mds460. Epub 2012 Oct 30 PMID: 23110807. [DOI] [PubMed] [Google Scholar]
- 18.Spermon J.R., Witjes J.A., Kiemeney L.A. Difference in stage and morphology-adjusted survival between young and elderly patients with a testicular germ cell tumor. Urology. 2002;60(5):889–893. doi: 10.1016/s0090-4295(02)01886-1. PMID: 12429322. [DOI] [PubMed] [Google Scholar]