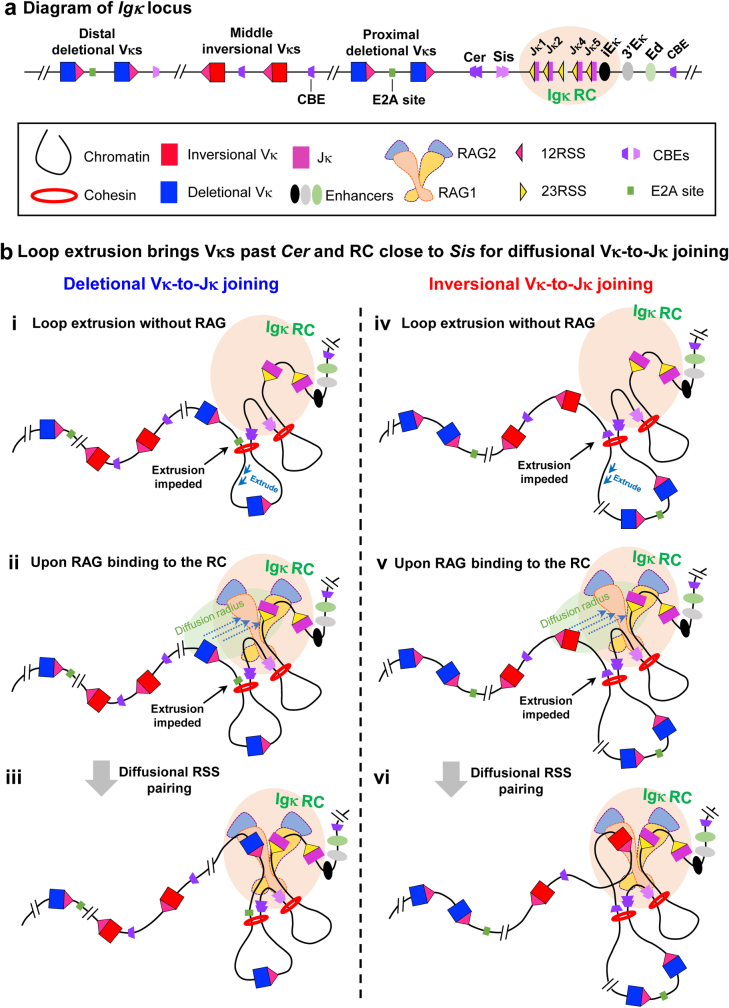
Extended Data Fig. 9. Working model for short-range diffusion-mediated primary Igκ V(D)J recombination.
a, Diagram of Igκ (not to scale). Elements and proteins illustrated are indicated in the box. b, Working model. (i) Loop extrusion of downstream chromatin through a cohesin ring impeded in the upstream direction at Sis juxtaposes the RC, a downstream impediment, to Sis. Simultaneously, loop extrusion of upstream chromatin through a cohesin ring impeded in the downstream direction at Cer brings the Vκ locus past Cer. (ii) During extrusion past Cer, relatively weak extrusion impediments, including CBEs and E2A sites (illustrated) across the Vκ locus dynamically impede extrusion at Cer, providing more opportunity for Vκ-RSSs to remain in short-range diffusion distance for interactions with RC-bound RAG. (iii) Binding of paired strong Vκ-RSSs to the RAG-1 active site across from strong Jκ-RSSs promote robust cleavage and/or joining. (iv-vi) Only a fraction of Vκ-RSSs brought into diffusion range pair with Jκ-RSSs, allowing extrusion to continue upstream where impediments slow down extrusion past Cer, providing opportunity for additional Vκ-RSSs to interact with RC-bound RAG. These panels diagram use of inversional-oriented Vκs, which can interact by the same short-range diffusion process outlined for deletional Vκs. The diagram is simplified to provide a general overview of the proposed mechanism, for which details await high resolution studies. Due to relatively weak Igκ impediments, this model is compatible with cohesin loading across the Vκ locus2. Also, RAG is likely not continuously bound to the RC2,7, allowing extrusion to continue past Cer. These latter features could allow active RAG-bound RCs to initiate the process at different points across the Vκ locus to optimize diverse Vκ utiliation2,7. Human Igκ, which undergoes deletional and inversional joining, has Cer-Sis-like elements in the Vκ-Jκ interval12,49,50 and high Vκ-RSS RIC scores33, consistent with employing a similar primary rearrangement mechanism to mouse Igκ.