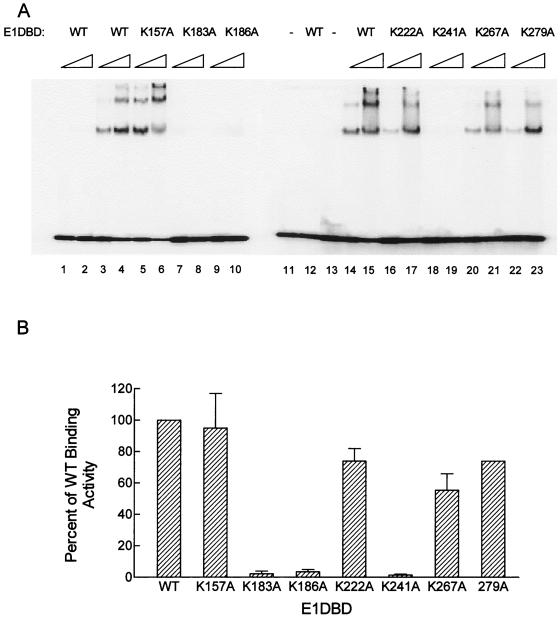
FIG. 3.
DNA binding by WT and mutant E1DBD proteins. (A) Gel mobility shift assays of WT and mutant E1DBD proteins. Each protein was assayed at 37.5 and 150 ng. Lanes marked − had no E1DBD added to the reaction and show the input unbound substrate; lanes 1, 2, 11, and 12 contain reactions using the E1BS0 substrate that lacks the E1BS, while all the other lanes have the complete origin substrate, E1BS1-4. (B) Quantitation of the binding results from panel A. The total amount of bound oligonucleotide was determined for the 37.5- and 150-ng samples with a PhosphorImager. The value for WT E1DBD was set as 100%, and the others are expressed relative to this value. Shown are the average results for the 150-ng samples from two experiments; error bars for the WT and 279A samples are too small to be seen. The relative results were similar with the 37.5-ng samples (not shown).