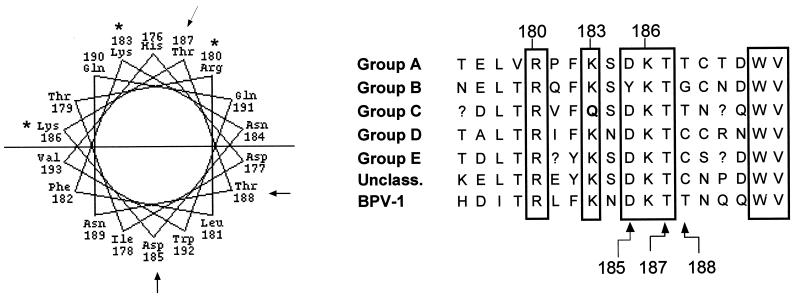
FIG. 6.
Helical projection and conservation of amino acids in HR1. Amino acids 176 to 193 of BPV E1 protein are displayed on the left in a helical wheel projection. The horizontal line divides the helix into two halves: an upper, highly basic, hydrophilic face, and a lower, more hydrophobic face. Lysine and arginine residues shown to be critical for DNA binding activity are marked with asterisks. The arrows indicate the two consecutive threonines and the aspartic acid that are functionally evaluated in Fig. 7. On the right is a comparison of the BPV E1 sequence from amino acids 176 to 193 aligned with the corresponding regions from the papillomavirus groups A to E and unclassified. The sequence shown for each papillomavirus group is the consensus sequence for these BPV E1-equivalent regions. Numbered residues refer to the BPV E1 amino acid number. Boxes indicate residues that are absolutely conserved with the exception of positions 183 and 185, which diverge in the group C and group B sequences, respectively.