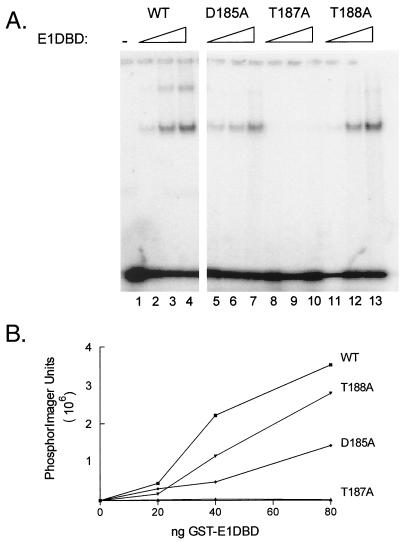
FIG. 7.
DNA binding activity of aspartic acid 185, threonine 187, and threonine 188 mutants. (A) D185, T187A, and T188A mutations were constructed into the E1DBD protein. Purified mutant and WT proteins were tested for origin binding activity by the mobility shift assay described in the legend to Fig. 3 with the complete origin oligonucleotide, E1BS1-4, as the substrate. The series of three lanes for each sample contained 20, 40, and 80 ng of protein. The lane marked − lacked E1DBD in the binding reaction. (B) The total of bound complexes for each sample in panel A was quantitated with a PhosphorImager and plotted as a function of protein amount.