Abstract
Background
Patients with grade 2 glioma exhibit highly variable survival. Re-irradiation for recurrent disease has limited mature clinical data. We report treatment results of pulsed reduced-dose rate (PRDR) radiation for patients with recurrent grade 2 glioma.
Methods
A retrospective analysis of 58 patients treated with PRDR from 2000 to 2021 was performed. Radiation was delivered in 0.2 Gy pulses every 3 minutes encompassing tumor plus margin. Survival outcomes and prognostic factors on outcome were Kaplan–Meier and Cox regression analyses.
Results
The median survival from the date of initial surgery was 8.6 years (95% CI: 5.5–11.8 years). 69% of patients showed malignant transformation to grade 3 (38%) or grade 4 (31%) glioma. Overall survival following PRDR was 12.6 months (95% CI: 8.3–17.0 months) and progression-free survival was 6.2 months (95% CI: 3.8–8.6 months). Overall response rate based on post-PRDR MRI was 36%. In patients who maintained grade 2 histology at recurrence, overall survival from PRDR was 22.0 months with 5 patients remaining disease-free, the longest at 8.2 and 11.4 years. PRDR was generally well tolerated.
Conclusions
To the best of our knowledge, this is the largest reported series of patients with recurrent grade 2 gliomas treated with PRDR radiation for disease recurrence. We demonstrate promising survival and acceptable toxicity profiles following re-irradiation. In the cohort of patients who maintain grade 2 disease, prolonged survival (>5 years) is observed in selected patients. For the entire cohort, 1p19q codeletion, KPS, and longer time from initial diagnosis to PRDR were associated with improved survival.
Keywords: brain tumor, glioma, outcomes, pulsed reduced-dose rate, radiation
Key Points.
Pulsed reduced-dose rate (PRDR) re-irradiation is safe and generally well tolerated.
Prognosticators: KPS, 1p19q codeletion, grade change, and time from diagnosis to PRDR.
Prolonged survival can be seen with PRDR in those remaining grade 2 at recurrence.
Importance of the Study.
To the best of our knowledge, this is the largest reported series of patients with recurrent grade 2 gliomas treated with pulsed reduced-dose-rate (PRDR) radiation for disease recurrence. The potential benefit from PRDR is highlighted in the demonstrated median survival of 12.6 months along with the acceptable toxicity profiles. Specific prognostic factors identified in multivariate analyses can be used in personalized patient management. Moreover, the study’s insights into the safety and tolerability of PRDR offer a promising palliative option for patients, potentially improving their quality of life.
Future implications include the potential for integrating PRDR with novel therapies, like IDH inhibitors, for enhanced patient outcomes. Such synergistic approaches could pave the way for innovative treatments in managing low-grade gliomas. The study also hints at the need for further genetic analysis to refine patient selection for PRDR, which could lead to more tailored and effective treatment protocols.
Approximately 93 500 new cases of primary brain and other Central Nervous System (CNS) tumors are diagnosed annually in the United States according to the Central Brain Tumor Registry of the United States.1 Low-grade gliomas account for roughly 15% of malignant CNS tumors and it is estimated that there are approximately 4000 new cases annually.2 Prior to 2016, World Health Organization (WHO) grading was based on the presence of specific histologic features: Mitoses, endothelial proliferation, nuclear atypia, and necrosis. In 2016, WHO CNS classification was updated to include molecular markers to better define CNS tumors.3 Again in 2021, the WHO further refined its classification of brain tumors by advancing the role of molecular diagnostics while maintaining integration of established histologic features and molecular markers.4
While grade 2 gliomas are considered to be low-grade, unfortunately, the vast majority eventually progress.5–7 These resultant grade 3 or 4 gliomas are then defined as high-grade gliomas. Despite a multimodal treatment paradigm including maximal safe surgical resection and either observation, radiotherapy (RT), chemotherapy, or combined chemoRT, the vast majority of patients recur at the original site.5,6 The time course for recurrence can span nearly a decade; however, at recurrence, up to 70% of LGGs have undergone malignant transformation to high-grade gliomas.8 When medically operable, initial management at recurrence typically involves re-resection. Treatment modalities for unresectable recurrent gliomas are more limited, especially since the majority recur locally in an area of previously irradiated tissue. Chemotherapy efficacy has been limited due to drug resistance, poor drug distribution in the tumor, and systemic toxicity.9,10 Re-irradiation with conventional techniques has shown promise regarding palliative outcomes; however, concerns regarding toxicity to normal tissue remain significant.
Pulsed reduced-dose-rate (PRDR) radiotherapy (RT) is a technique for altering the radiation delivery dose rate by employing intrafraction temporal dose modulation to arrive at an effective low-dose rate for the entire treatment fraction.11–18 As compared to conventional RT, in which a dose of ~2 Gy is delivered at a dose rate of 4–6 Gy/min, when employing intrafraction temporal dose modulation the dose per fraction is broken up into multiple 0.2 Gy pulses delivered at the start of a given 3-minute interval resulting in an effective dose rate of 0.067 Gy/min18. The reduced-dose rate technique exploits a fundamental difference between normal and malignant cells, limiting toxicity to normal tissue via improved sublethal damage repair at low-dose rates and decreased TGF-B release.11,12,19 PRDR RT may also have therapeutic advantages in terms of tumor control owing to the phenomenon of low-dose hyper-radiosensitivity (LDHRS), in which cells display higher radiosensitivity to lower doses of radiation (0.3–0.5 Gy).18 LDHRS has been demonstrated in several model systems.13,14,20–23 Another potential advantage is that it can be delivered at virtually any radiation therapy center without the need for specialized techniques such as hyperthermia or brachytherapy, and can readily treat deep-seated recurrent tumors.
Materials and Methods
Patient Selection and Characteristics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Fifty-eight consecutive patients from 2001 to 2022 were identified from an institutional database who were initially diagnosed with grade 2 glioma and ultimately underwent re-irradiation with pulse reduced-dose rate radiation therapy. An attempt was made to reclassify the patient cohort to the WHO 2021 system; however, due to a majority of the pathology dating over 10 years old, molecular data required for reclassification was unavailable.
PRDR Characteristics and Technique
Median re-irradiation dose with PRDR was 54 Gy (range, 2–60 Gy) using 0.2 Gy pulses every 3 minutes creating an apparent dose rate of 0.0667 Gy/min. Most commonly, a 3D conformal, 3-field technique was used, encompassing gross disease with a 1–2 cm margin. While intensity-modulated radiation therapy has been used more frequently in the past decade, this still represents a minority of the total patient cohort. Treatment was delivered with 6-MV photons. Customized aquaplast head and neck masks were used to provide optimal immobilization and reproducibility. Planning target volumes were determined with MRI findings, including the contrast-enhancing lesion and surrounding T2-weighted or fluid-attenuated inversion recovery abnormality if present. A 2.0 cm margin was added to establish the CTV.
The dose constraints were determined from the PRDR plan only, irrespective of the isodose distributions of the initial RT plan. The lens and cervical spine were always shielded from the direct beam. Attempts were made to limit the retreatment dose to the optic chiasm to 54 Gy, the retina of at least one eye to 50 Gy, and the brainstem to 54 Gy. No limitations were placed on the cumulative dose, including the initial treatment dose.
Follow-up and Statistical Analysis
After completion of therapy, the response rate was defined by MRI imaging, improvement in KPS, reduction of pretreatment dexamethasone, and improvement of any pretreatment symptoms. Recurrence was defined namely via progression on follow-up MRI imaging. MRI scans were obtained according to routine follow-up parameters or when clinical worsening developed. Patients were routinely seen for short interval follow-up 3–6 weeks following completion of PRDR, then in 2.5–3 month intervals with MRI or as clinically indicated. Follow-up visits included MRI with contrast and a thorough neurologic examination.
Data collection and analysis were conducted in accordance with an institutional review board-approved retrospective review process. The analysis was aimed to ascertain survival estimates over time and to identify factors independently influencing survival outcomes. The timeframe for overall survival (OS) was computed from the initial diagnoses from initial diagnosis until the date of last follow-up or recorded date of mortality. In the event no date of mortality was found in the chart, OS was augmented using social security death searches for patients lost to follow-up. Similarly, PRDR survival was calculated from the PRDR initiation date. Survival functions were estimated using the Kaplan–Meier method, and survival disparities among categorical variables were assessed through log-rank testing. Influence of prognostic factors on outcome was evaluated using univariate and multivariate Cox proportional regression models. Statistical assessment and graphical analysis were done using IBM SPSS Statistics, Version 28, Armonk, NY.
Results
Overall, re-irradiation using PRDR was well tolerated. Intended treatment was completed in all patients except for those who experienced symptomatic disease progression (n = 5) during treatment, had medical issues limiting continuation (n = 1), or elected to discontinue their treatment course (n = 3).
Patient Demographics and Tumor Characteristics
Patient characteristics including age, initial histology, tumor location based on MRI, tumor grade at recurrence, and tumor marker findings are outlined in Table 1. Median age at PRDR was 42.9 years (range, 25–75) with 66% of patients receiving PRDR before they were 50 years old. Recurrence sites varied with regard to location and severity; 29 patients with bilateral recurrence or corpus callosum invasion, 10 patients with brainstem or thalamus invasion, and 5 patients with midline deviation or herniation. Initial histology revealed 29 Astrocytomas, 23 Oligodendrogliomas, and 6 Oligoastrocytomas. MGMT methylation was positively identified in 13 patients, IDH mutation in 15 patients, and 1p/19q codeletion in 16 patients. Many patients underwent their initial brain tumor surgery in the 1990s-early 2000s at outside facilities, therefore initial MGMT methylation, IDH mutation, and 1p19q codeletion status were only available in 60%, 46%, and 64% of patients, respectively (Table 1).
Table 1.
Patient and Tumor Characteristics
| Age at initial diagnosis (y) | ||
|---|---|---|
| Median | 43 | |
| Range | 25–75 | |
| KPS prior to PRDR (n) | ||
| 100 | 16 (28%) | |
| 90 | 22 (38%) | |
| 80 | 12 (21%) | |
| 70 | 2 (3%) | |
| 60 | 2 (3%) | |
| 50 | 4 (7%) | |
| Bilateral hemisphere or corpus callosum involvement (n) | 29 (50%) | |
| Brainstem or thalamus involvement (n) | 10 (17%) | |
| Herniation or midline shift (n) | 5 (9%) | |
| Histology (n) | Astrocytoma | 29 (50%) |
| Oligodendroglioma | 23 (40%) | |
| Oligoastrocytoma | 6 (10%) | |
| Tumor markers at the time of diagnosis (n) | ||
| MGMT methylation | Yes | 13 (22%) |
| No | 22 (38%) | |
| Unknown | 23 (40%) | |
| IDH mutated | Yes | 15 (26%) |
| No | 12 (21%) | |
| Unknown | 31 (53%) | |
| 1p/19q co-deleted | Yes | 16 (28%) |
| No | 21 (36%) | |
| Unknown | 21 (36%) | |
| Grade at time of recurrence (n) | ||
| No change (grade 2) | 18 (31%) | |
| Grade 3 | 22 (38%) | |
| Grade 4 | 18 (31%) | |
Characteristics at Time of Recurrence and Treatments Rendered
Treatment summaries are provided in Table 2, describing the number of surgeries, median initial radiation dose, and dose with re-irradiation, and use and timing of chemotherapy.
Table 2.
Treatment Details
| Initial surgery (n) | ||
|---|---|---|
| Gross total resection (GTR) | 19 (33%) | |
| Subtotal resection (STR) | 24 (41%) | |
| Biopsy only | 12 (21%) | |
| Unknown | 3 (5%) | |
| Number of resections (n) | ||
| 1 | 10 (17%) | |
| 2 | 31 (54%) | |
| 3+ | 17 (29%) | |
| Initial RT dose (Gy) | Median | 54 |
| Range | 50–70 | |
| PRDR re-irradiation dose (Gy) | Median | 54 |
| Range | 2–60 | |
| Average cumulative dose (Gy) | 98.3 | |
| Systemic therapy timing (n) | Initial concurrent CRT | 16 (28%) |
| Adjuvant | 17 (29%) | |
| At the time of progression | 36 (62%) | |
| Concurrent with PRDR | 10 (17%) | |
| Following PRDR | 10 (17%) | |
On initial presentation, 19 patients underwent a gross total resection (GTR), 24 subtotal resections (STR), 12 biopsy only, and 3 with poorly defined histologies regarding initial surgery. All patients re-treated with PRDR had been previously treated with RT using a standard dose rate to a median dose of 54 Gy (range, 50.4–70 Gy). Median re-irradiation dose of 54 Gy was delivered ranging from 2 to 60 Gy. Thirty-six patients received planned 54 Gy, 8 patients received whole brain PRDR at lower median total dose (dose ranging from 36 to 54 Gy), and 14 patients were treated to doses other than 54 ranging from 2 to 60 Gy. Sixteen patients received concurrent chemotherapy with initial RT, 17 received adjuvant chemotherapy, and 36 received chemotherapy during a prior tumor progression. Ten patients received concurrent chemotherapy with PRDR RT (Temodar [5] or Avastin [5]). 83% (48/58) underwent at least a second sub- or GTR and 93% of patients received chemotherapy at some point during their overall treatment course (54/58).
At recurrence, 18 patients remained in grade 2, whereas 40 progressed to grade 3 (22/58) or grade 4 (18/58) as outlined in Table 1. On recurrence, patients were promptly evaluated in a multidisciplinary neuro-oncology clinic. Patients were treated according to institutional practice involving re-operation when feasible and considered for systemic therapy options including temozolomide, procarbazine, cyclophosphamide, vincristine, carmustine, lomustine, dalteparin, carboplatin, irinotecan, hydroxyurea, high-dose tamoxifen, signal transduction inhibitors, histone deacetylase inhibitors, antiangiogenic agents, and clinical trial options available at that given time. Patients were heavily pretreated prior to PRDR with all patients undergoing at least some form of salvage surgical and/or systemic therapy prior to re-irradiation. When all therapy options were exhausted or declined by the patient, patients were considered for PRDR. Ten patients underwent a single surgical resection, 31 had 2 resections, and 17 had 3 or more resections (Table 2). Twenty-eight patients underwent surgery within 4 months of PRDR, 15 of whom had GTR prior to re-irradiation with PRDR.
Survival Analyses
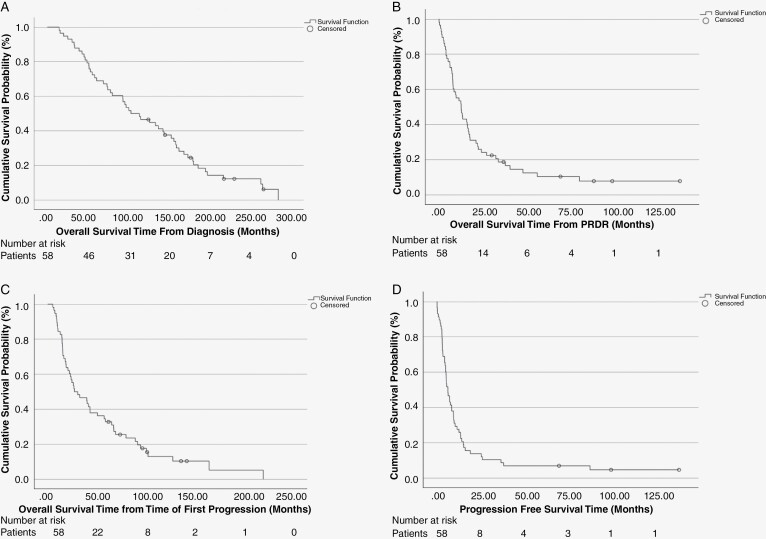
The overall survival (OS) for the entire cohort from the time of initial diagnosis is depicted in Figure 1A, showing a median OS of 8.6 years (95% CI: 5.5–11.8 years). Figure 1B illustrates the OS from the time of re-irradiation with PRDR showing median OS of 12.6 months (95% CI: 8.3–17.0 months). Figure 1C depicts the overall survival from first documented progression with median overall survival of 27.0 months (95% CI: 8.3–45.9). Progression-free survival (PFS) from PRDR is demonstrated in Figure 1D, with a median PFS of 6.2 months (95% CI: 3.8–8.6 months).
Figure 1.
(A) Overall survival (OS) for the entire cohort taken from the time of initial diagnosis. (B) Overall survival (OS) for all 58 patients following re-irradiation with pulsed reduced-dose rate (PRDR). (C) Overall survival (OS) for all 58 patients from the time of first documented progression following initial intervention. (D) Progression-free survival (PFS) for all 58 patients from time of PRDR.
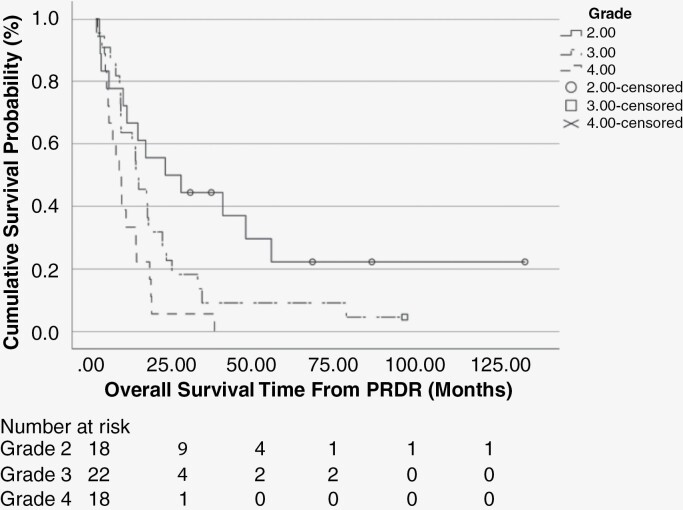
When stratified by recurrent tumor grade (Figure 2) median overall survival was 22.0 months (95% CI: 0.0–45.44 months) for patients maintaining grade 2 tumors, 12.6 months (95% CI: 7.3–17.9 months) for grade 3 tumors, and 7.3 months (95% CI: 3.7–10.8 months) for grade IV tumors. The 1-, 2- and 5-year actuarial survival rate was 67%, 50%, and 22% for those with grade 2 tumors, 59%, 23%, and 9% for those with grade 3 tumors, and 33%, 6%, and 0% for those with grade 4 tumors respectively. Six patients in the 58-patient cohort remain alive, the 2 longest term survivors at 8.2 and 11.4 years from their PRDR treatment.
Figure 2.
Overall survival (OS) for all 58 patients from time of pulsed reduced-dose rate, stratifying for grade at time of progression; those that remained grade 2, transformed to grade 3, or transformed to grade 4.
Table 3 and Table 4 outline the results from multivariate analysis for the patient cohort identifying several features of significance with respect to OS and PFS from PRDR. The features that maintain significance in multivariate analysis for OS include: KPS < 80 (P < .001), 1p19q codeletion (P = .004), grade transformation (.002), and interval > 5 years from initial surgery to time of first progression (P = .008). There were additional factors found to be significant in univariate analysis that did not maintain significance in multivariate analysis such as patients < 40 years old at time of diagnosis had worse OS (P = .031), patients on Dexamethasone at the start of PRDR had worse OS (P = .003), and patients who underwent surgery within 4 months of PRDR had better OS (P = .041).
Table 3.
Multivariate Model for Overall Survival From Initiation of Pulsed Reduced-Dose Rate
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| KPS (< 80, n = 9; vs. ≥ 80, n = 49) | 16 | 3.9–65.6 | <.001 |
| 1p191 codeletion status (Co-deleted, n = 16; vs. non-co-deleted, n = 20) | 0.3 | 0.1–0.7 | .004 |
| Histologic grade transformation (remain grade 2, n = 18; vs. transformed to grade 3 or 4, n = 40) | 4.2 | 1.7–10.4 | .002 |
| Time from diagnosis to first progression (< 5 years, n = 28; vs. ≥ 5 years, n = 30) | 3.1 | 1.3–7.1 | .008 |
Table 4.
Multivariate Model for Progression-Free Survival from Pulsed Reduced-Dose Rate
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| KPS decrease during PRDR (decrease, n = 22; vs. same or increase, n = 36) | 2.4 | 1.3–4.3 | .006 |
| Time from diagnosis to first progression (< 5 years, n = 28; vs. ≥ 5 years, n = 30) | 2.2 | 1.2–3.8 | .008 |
| Surgery within 4 months of PRDR (within 4 months, n = 28; vs. ≥ 4 months or no surgery, n = 30) | 0.5 | 1.7–10.4 | .018 |
With regards to PFS following PRDR, age less than 40 years old, Dexamethasone at time of PRDR, time to first progression of less than 5 years, surgery within 4 months of PRDR, and a decrease in KPS during course of PRDR were all significant on univariate analysis. The features that maintain significance in multivariate analysis for PFS include: Surgery within 4 months of PRDR (P = .018), KPS decrease during course of PRDR (P = .006) and interval > 5 years prior to PRDR (P = .008).
Overall, treatment was well tolerated by the patient cohort. Fatigue (n = 21), alopecia (n = 11) and headaches (n = 10) were found to be the most common acute toxicities whereas short-term memory changes (n = 6) were the most common late toxicity. Five patients had documented seizures during treatment and one patient stopped after a single fraction due to cerebral edema. Conversely, 2 patients had documented decreased frequency of seizure-like activity during PRDR and 4 patients had improved KPS during the PRDR course.
Discussion
Despite the potential for long-term survival for patients with grade 2 glioma following initial diagnosis and treatment, ultimate tumor recurrence remains a significant challenge. GTR inevitably becomes difficult due to anatomic location, the infiltrative nature of gliomas, and patient performance status and comorbidities. Seventy percent of recurrent grade 2 tumors will show progression in grade thus signifying a more aggressive clinical picture and worse prognosis.8 Systemic chemotherapy options have often already been employed and modest evidence exists indicating additional efficacy for ongoing systemic therapy with regard to tumor control, OS, and PFS. Due to gliomas predominantly recurring in areas close to the primary site, radiation must be applied cautiously to reduce the risk of significant normal tissue damage in previously irradiated locations. For many patients, PRDR affords a viable option to consider in an effort to prolong survival and maintain quality of life.
The present data supports the concept that re-irradiation using PRDR is a safe and effective means to provide a palliative option for select patients with recurrent gliomas. For the 58 patients in this study initially diagnosed with low-grade glioma, PRDR re-irradiation led to a median overall survival of 12.6 months and was well tolerated with regard to toxicity profile.
For LGGs, prognostic factors are not clearly defined and various cooperative groups have described potential risk factors differently. Age, histology, tumor size, pre-operative neurologic deficits, and resection status are among the poor prognostic factors identified.24–26 Using a multivariate analysis outlined in Table 3, we found 4 factors to be significant prognosticators for OS following PRDR: KPS, 1p19q codeletion status, grade transformation, and an interval of more than 5 years from initial surgery to first progression. While univariate analysis showed younger patients (age < 40) at time of diagnosis fared worse, this was not borne out in the multivariate analysis. Similarly, univariate significance was noted in patients undergoing surgery within 4 months prior to PRDR, but this did not hold multivariate significance for OS post-PRDR. This factor was significant in multivariate analysis for PFS; however, indicating a possible sustainability in quality of life but not prolongation of life with PRDR. Multivariate significance was found for PFS in patients undergoing surgery within 4 months prior to PRDR, an interval of more than 5 years from initial surgery to first progression, and patients with decreased KPS during PRDR.
The analysis by Combs et al. provided a useful comparator for re-irradiation of low-grade gliomas.27 This investigation analyzed the effectiveness of stereotactically guided fractionated re-irradiation for patients with recurrent gliomas at primary diagnosis. Results were stratified by glioblastoma, grade 3 glioma, and grade 2 glioma based on WHO classification at that time. For 71 patients with grade 2 gliomas, median overall survival from initial diagnosis and time from initial radiation to re-irradiation was found to be 111 months and 50 months respectively. Comparatively, this investigation showed median overall survival from the initial diagnosis to be 103 months and time from initial radiation to re-irradiation to be 60 months. The Combs study showed a longer median survival from re-irradiation as well as longer median PFS from re-irradiation (23 vs. 12.6 months and 12 vs. 6.3 months, respectively). No investigation into grade transformation was noted in this publication but OS curves comparing Combs grades 2, 3, and 4 glioma versus the current patient cohort who remained grade 2, transformed to grade 3, and transformed to grade 4 appear nearly identical (median OS of 22, 12, and 8 months vs. median OS of 22, 12, and 7 months, respectively). Patient characteristics were similar between cohorts with the main differences being a lesser percentage of patients with oligodendroglioma histology in the Combs study (9.5% vs. 40%) and age at diagnosis of patients older than 50 y/o (12.7% vs. 32%). Although a smaller patient cohort than Combs, Lee et al. retrospectively reviewed 36 patients with recurrent gliomas who received re-irradiation. In this cohort, 17 patients had grade 2 glioma at initial diagnosis, of which 7 remained grade 2 at the time of re-irradiation. Overall PFS and OS were found to be 8 and 11 months respectively. On multivariate analysis, grade 2 histology at the time of recurrence was not prognostic for improved survival.28 While small numbers in this review limit the findings, it does emphasize the variability in survival in this patient population.
Clinical reports of PRDR and similar semi-continuous low-dose-rate irradiation in the treatment of recurrent gliomas across grades underscore the efficacy and tolerability observed in this analysis. Schultz and Geard’s pioneering work elucidated the radioresponse of human gliomas to both acute and chronic irradiation, laying the groundwork for treatment strategies based on tumor grade.29 Building on this foundation, Siker et al conducted a phase I/II study emphasizing the potential of the approach in improving prognosis, particularly in cases resistant to conventional treatments.30 Overall, these findings underscore the promise of exploring innovative radiation techniques such as PRDR in the management of recurrent gliomas.
Limitations of the current study include those commonly associated with a retrospective analysis. Patients were not randomly selected for PRDR and the patient population may have had a different performance status than the general population of patients with recurrence. Patients with very poor performance status may have been referred directly to hospice rather than radiation oncology for consideration of retreatment. Additionally, tumor genetics may play a valuable role in prognostication, however, a large portion of our patient cohort had unknown MGMT and IDH information. Gene sequencing from frozen brain pathology specimens taken pre- and post-PRDR may provide additional insight into patients who may benefit from additional radiation. Finally, retrospective cohorts can underestimate toxicity when data are compiled from hospital charts rather than from standardized adverse event surveys.
Lastly, recent findings provide growing support for IDH inhibitors in grade 2 IDH-mutant glioma with regards to PFS advantage and postponement of subsequent treatment interventions.31 An area warranting further exploration includes the investigation of the combined use of IDH inhibitors and PRDR in this patient population.
Conclusion
Pulsed reduced-dose-rate re-irradiation is a strategy that is generally well tolerated in patients with recurrent grade 2 gliomas, thereby affording a viable palliative option to prolong survival and maintain quality of life. KPS, 1p/19q codeletion, grade transformation, and an interval of >5 years between radiation treatments were significant prognosticators for survival following PRDR re-irradiation on multivariate analysis.
Acknowledgments
We extend our deepest gratitude to all patients who participated in this study. Their willingness to contribute to medical research is invaluable to advancing our knowledge for the future.
Contributor Information
Colin M Harari, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Adam R Burr, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Brett A Morris, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Wolfgang A Tomé, Department of Radiation Oncology, Montefiore Medical Center, Bronx, New York, USA.
Adam Bayliss, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Ankush Bhatia, University of Wisconsin Carbone Cancer Center, Madison, Wisconsin, USA.
Patrick T Grogan, Moffitt Cancer Center, Tampa, Florida, USA.
H Ian Robins, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Steven P Howard, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Carbone Cancer Center, Madison, Wisconsin, USA.
Conflict of interest statement
C.M.H., B.A.M., W.A.T., A.B., P.T.G., H.I.R., and S.P.H. report no conflicts of interest. A.R.B. reports research funding from GE Healthcare and a grant from Siemens. A. Bhatia reports consulting fees from Servier Advisory Board, payment or honoraria from Wisconsin Association of Hematology and Oncology, and service as an Editor for Current Oncology Reports and Medlink Neurology.
Authorship statement
Data analysis and writing of the manuscript: C.M.H., A.R.B., B.A.M., W.A.T., A.B., A.B., P.T.G., H.I.R., and S.P.H.. No unpublished papers were cited.
Data availability
Research data are stored in an institutional repository and will be shared upon request with the corresponding author.
References
- 1. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro-Oncology. 2022;24(suppl_5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol (Berl). 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. Neuro-Oncology. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salcman M, Scholtz H, Kaplan RS, Kulik S.. Long-term survival in patients with malignant astrocytoma. Neurosurgery. 1994;34(2):213–9; discussion 219. [DOI] [PubMed] [Google Scholar]
- 6. Durmaz R, Erken S, Arslantaş A, et al. Management of glioblastoma multiforme: With special reference to recurrence. Clin Neurol Neurosurg. 1997;99(2):117–123. [DOI] [PubMed] [Google Scholar]
- 7. Adkison JB, Tomé W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):835–841. [DOI] [PubMed] [Google Scholar]
- 8. van den Bent M, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 9. Brandes AA, Fiorentino MV.. The role of chemotherapy in recurrent malignant gliomas: An overview. Cancer Invest. 1996;14(6):551–559. [DOI] [PubMed] [Google Scholar]
- 10. McBain C, Lawrie TA, Rogozińska E, et al. Treatment options for progression or recurrence of glioblastoma: A network meta-analysis. Cochrane Database Syst Rev. 2021;2021(1):CD013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marples B, Collis SJ.. Low-dose hyper-radiosensitivity: Past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70(5):1310–1318. [DOI] [PubMed] [Google Scholar]
- 12. Meyer JE, Finnberg NK, Chen L, et al. Tissue TGF-β expression following conventional radiotherapy and pulsed low-dose-rate radiation. Cell Cycle. 2017;16(12):1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Short SC, Kelly J, Mayes CR, Woodcock M, Joiner MC.. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int J Radiat Biol. 2001;77(6):655–664. [DOI] [PubMed] [Google Scholar]
- 14. Joiner MC, Marples B, Lambin P, Short SC, Turesson I.. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49(2):379–389. [DOI] [PubMed] [Google Scholar]
- 15. Richards GM, Tomé WA, Robins HI, et al. Pulsed reduced dose-rate radiotherapy: A novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009;114(2):307–313. [DOI] [PubMed] [Google Scholar]
- 16. Pierquin B, Calithchi E, Mazeron JJ, Le Bourgeois JP, Leung S.. Update on low dose rate irradiation for cancers of the oropharynx-May 1986. Int J Radiation Oncol Biol Phys. 1987;13(2):259–261. [DOI] [PubMed] [Google Scholar]
- 17. Pierquin B, Tubiana M, Pan C, et al. Long-term results of breast cancer irradiation treatment with low-dose-rate external irradiation. Int J Radiat Oncol Biol Phys. 2007;67(1):117–121. [DOI] [PubMed] [Google Scholar]
- 18. Tomé WA, Howard SP.. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol. 2007;80(949):32–37. [DOI] [PubMed] [Google Scholar]
- 19. Hall EJ, Giaccia AJ.. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 20. Short SC, Mitchell SA, Boulton P, Woodcock M, Joiner MC.. The response of human glioma cell lines to low-dose radiation exposure. Int J Radiat Biol. 1999;75(11):1341–1348. [DOI] [PubMed] [Google Scholar]
- 21. Beauchesne PD, Bertrand S, Branche R, et al. Human malignant glioma cell lines are sensitive to low radiation doses. Int J Cancer. 2003;105(1):33–40. [DOI] [PubMed] [Google Scholar]
- 22. Short SC, Woodcock M, Marples B, Joiner MC.. Effects of cell cycle phase on low-dose hyper-radiosensitivity. Int J Radiat Biol. 2003;79(2):99–105. [PubMed] [Google Scholar]
- 23. Marin LA, Smith CE, Langston MY, Quashie D, Dillehay LE.. Response of glioblastoma cell lines to low dose rate irradiation. Int J Radiat Oncol Biol Phys. 1991;21(2):397–402. [DOI] [PubMed] [Google Scholar]
- 24. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 25. Reichenthal E, Feldman Z, Cohen ML, Loven D, Zucker G.. Hemispheric supratentorial low-grade astrocytoma. Neurochirurgia (Stuttg). 1992;35(1):18–22. [DOI] [PubMed] [Google Scholar]
- 26. Gorlia T, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: A pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro-Oncol. Neuro-Oncology. 2013;15(11):1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D.. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23(34):8863–8869. [DOI] [PubMed] [Google Scholar]
- 28. Lee J, Cho J, Chang JH, Suh CO.. Re-irradiation for recurrent gliomas: Treatment outcomes and prognostic factors. Yonsei Med J. 2016;57(4):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schultz CT, Geard CR.. Radioresponse of human astrocytic tumors across grade as a function of acute and chronic irradiation. Int J Radiation Oncol Biol Phys. 1990;19(6):1397–1403. [DOI] [PubMed] [Google Scholar]
- 30. Siker ML, Firat SY, Mueller W, Krouwer H, Schultz CJ.. Semicontinuous low-dose-rate teletherapy for the treatment of recurrent glial brain tumors: Final report of a phase I/II Study. Int J Radiat Oncol Biol Phys. 2012;82(2):765–772. [DOI] [PubMed] [Google Scholar]
- 31. Mellinghoff IK, van den Bent MJ, Blumenthal DT, et al. ; INDIGO Trial Investigators. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N Engl J Med. 2023;389(7):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request with the corresponding author.