Figure 2.
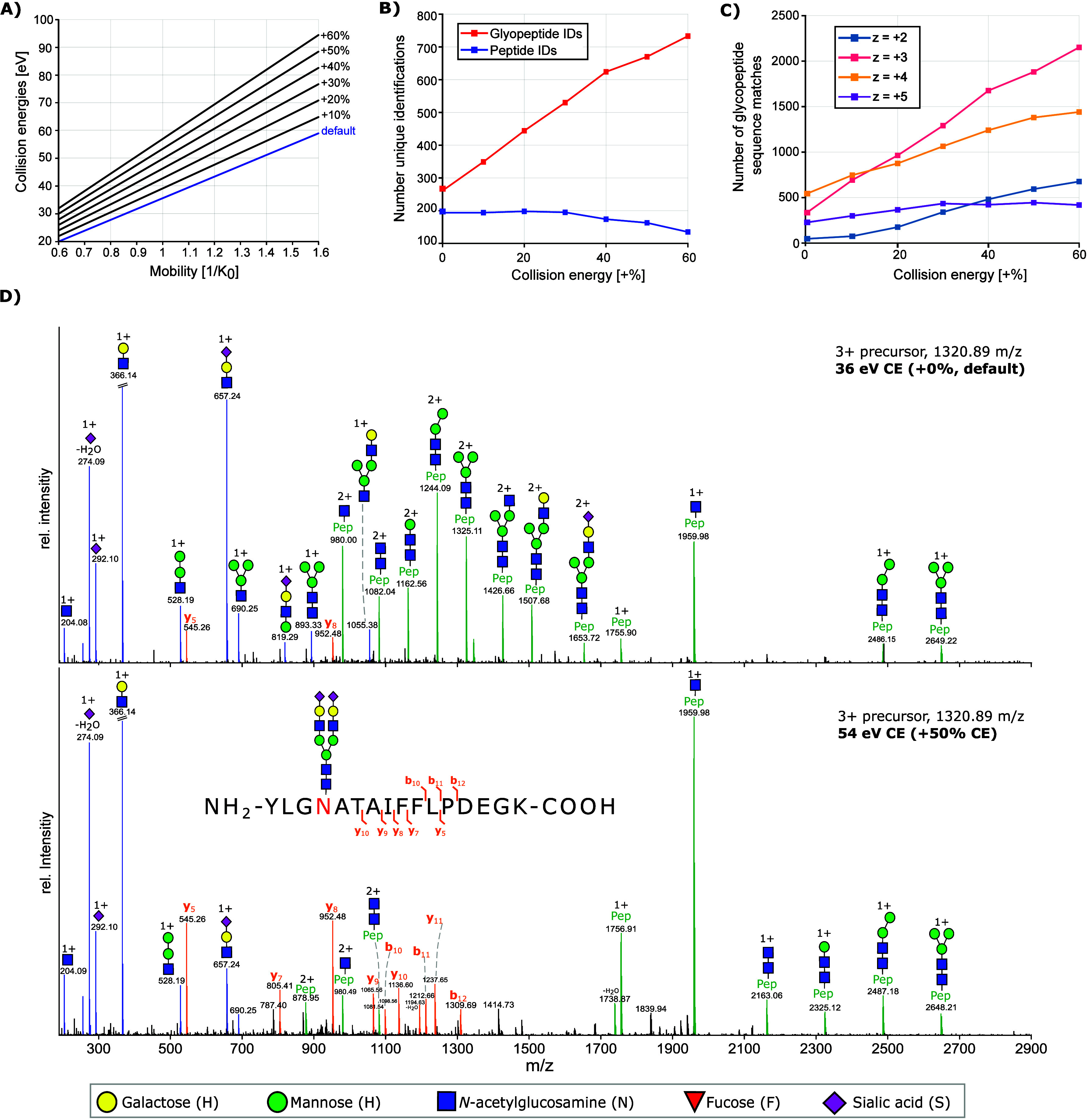
Collision energy optimization. (A) Collision energies are set as a function of ion mobility and increase linearly with higher mobility values. The slope and offset were increased in steps of 10% compared to the proteomics default settings to optimize collision energies for glycopeptide fragmentation. (B) Increasing the collision energies led to an increase in unique glycopeptide identifications from less than 300 to over 700 at higher CE values while peptide identifications decreased. (C) Ideal CE values depended on precursor charge state. Precursors with a lower charge state (+2, +3) benefited more from increased CE values compared to highly charge precursors (+4, +5). (D) Exemplary MS/MS spectrum of a +3 glycopeptide with standard proteomics CE values shows mainly the fragmentation of the glycan moiety as oxonium ions (blue) or glycan fragments with intact peptide residue (green). At higher CE values (+50%), more b- and y-ion fragments of the peptide backbone (orange) are observed to support peptide moiety identification by protein sequence database search algorithms. The annotation of glycan fragments represents only one possibility. Other isomers are plausible.
