Abstract
Key Clinical Message
Sarcoidosis, although predominantly affecting the lungs, can present with cirrhosis, posing diagnostic challenges. Elevated ACE levels and atypical liver enzyme patterns should prompt consideration of sarcoidosis in cryptogenic cirrhosis cases, necessitating comprehensive evaluation including liver biopsy and imaging for accurate diagnosis and timely management.
Abstract
Sarcoidosis is a systemic disease that can affect various organs, leading to a diverse range of clinical manifestations that make diagnosis challenging. Here, we present a case of sarcoidosis in a middle‐aged male who presented with cirrhosis. The cause of cirrhosis remained unknown for 4 years until the development of lymphadenopathy and ground‐glass opacities on lung imaging. A liver biopsy was performed, which revealed noncaseating granulomatous inflammation, thereby identifying sarcoidosis as the cause of cirrhosis. The patient was treated with oral steroids, which slightly improved his liver function over a short period. Given the diverse presentations of sarcoidosis, it should be considered as a possible differential diagnosis in cases of cryptogenic cirrhosis.
Keywords: ACE, cirrhosis, granuloma, sarcoidosis
1. INTRODUCTION
Sarcoidosis is a granulomatous disease of unknown etiology that can affect almost any organ. The prevalence is slightly higher in females and African Americans, with a peak onset age of 20–40 years. 1 , 2 , 3 It most commonly affects the lungs, followed by the lymph nodes. 2 Liver involvement is not uncommon in sarcoidosis (approximately 11.5%); however, about 80%–85% of cases are asymptomatic. 3 , 4 The usual pathology is granuloma formation in the liver, but the incidence of cirrhosis is less than 1%. 3 , 5 In this report, we describe the case of a middle‐aged male who presented with a 4‐year history of cirrhosis with no identifiable cause. The patient was subsequently diagnosed with sarcoidosis.
2. CASE HISTORY
A nonalcoholic, nondiabetic construction worker in his late thirties consulted a physician in 2018 complaining of pruritus. He also experienced significant weight loss (10 kg over 3 months). His liver enzymes were also elevated. Alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) were markedly raised (487 U/L and 88 U/L, respectively). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were slightly increased, but bilirubin was normal. With this cholestatic pattern of hepatic dysfunction, magnetic resonance cholangiopancreatography (MRCP) revealed multiple irregular strictures in the intrahepatic bile ducts. Unfortunately, the patient lost the film and could only provide reports. An abdominal computed tomography (CT) scan revealed liver cirrhosis with nonspecific splenic nodules (Figure 1). Based on these findings, the patient was diagnosed with liver cirrhosis secondary to primary sclerosing cholangitis (PSC). Although liver biopsy was recommended to confirm the diagnosis, the patient refused to undergo the procedure.
FIGURE 1.
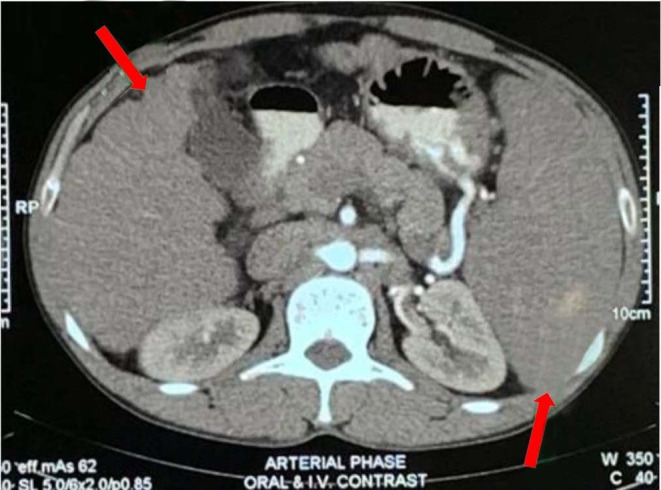
CT scan of abdomen showing nodular liver surface with heterogenous parenchymal attenuation and splenomegaly with nonspecific splenic nodule.
Over the course of the next 4 years, he was relatively well, with occasional itching and two episodes of melena. He underwent his first session of esophageal band ligation in 2020 and was administered propranolol (20 mg) twice daily. He did not undergo regular follow‐up.
Approximately 6 months prior, the patient presented with a history of hematemesis and melena for 3 days, along with an increase in the intensity of itching. Urgent endoscopy and a second session of esophageal band ligation were performed. There was no history of intravenous drug abuse, sexual promiscuity, previous surgery, features suggestive of inflammatory bowel disease, or family history of liver disease.
Examination revealed an oriented, cooperative male patient with a body mass index of 24 kg/m2. He was mildly anemic, but non‐icteric. There were two enlarged lymph nodes in the jugulodigastric region, both 1 × 1 cm, nontender, soft in consistency, mobile, not fixed to underlying structures, and with no discharging sinus. No clubbing, leukonychia, gynecomastia, or spider angioma were observed. However, the spleen was firm and nontender and markedly enlarged, about 15 cm from the left costal margin along its long axis. The liver was not palpable. Ascites was absent, and both testes were atrophied. Examination of other systems was unremarkable.
3. INVESTIGATIONS AND TREATMENT
Investigations revealed that his hemoglobin level was 6.9, that rose to 10.1 after giving two units of blood. AST and ALT levels were more than two times the upper limit of normal. ALP and GGT levels were elevated to 240 U/L and 148 U/L, respectively. Serum albumin and prothrombin time were normal.
As the cause of cirrhosis was not previously confirmed, we reassessed the patient. Viral markers for hepatitis B, C, and E and HIV were negative. Serum iron profile, serum ceruloplasmin, and 24‐hour urinary copper levels were normal. Autoantibody screens, including antinuclear antibody, anti‐mitochondrial antibody, smooth muscle antibody, and liver kidney microsomal antibody were all negative. IgG levels were within the normal range and p‐ANCA was negative. Corrected serum calcium levels were normal. Ultrasonography revealed features suggestive of liver cirrhosis, with a suspected space‐occupying lesion, splenomegaly, and mild ascites. Magnetic resonance imaging (MRI) with MRCP showed cirrhosis of the liver with splenomegaly without any intrahepatic bile duct abnormalities (Figure 2).
FIGURE 2.
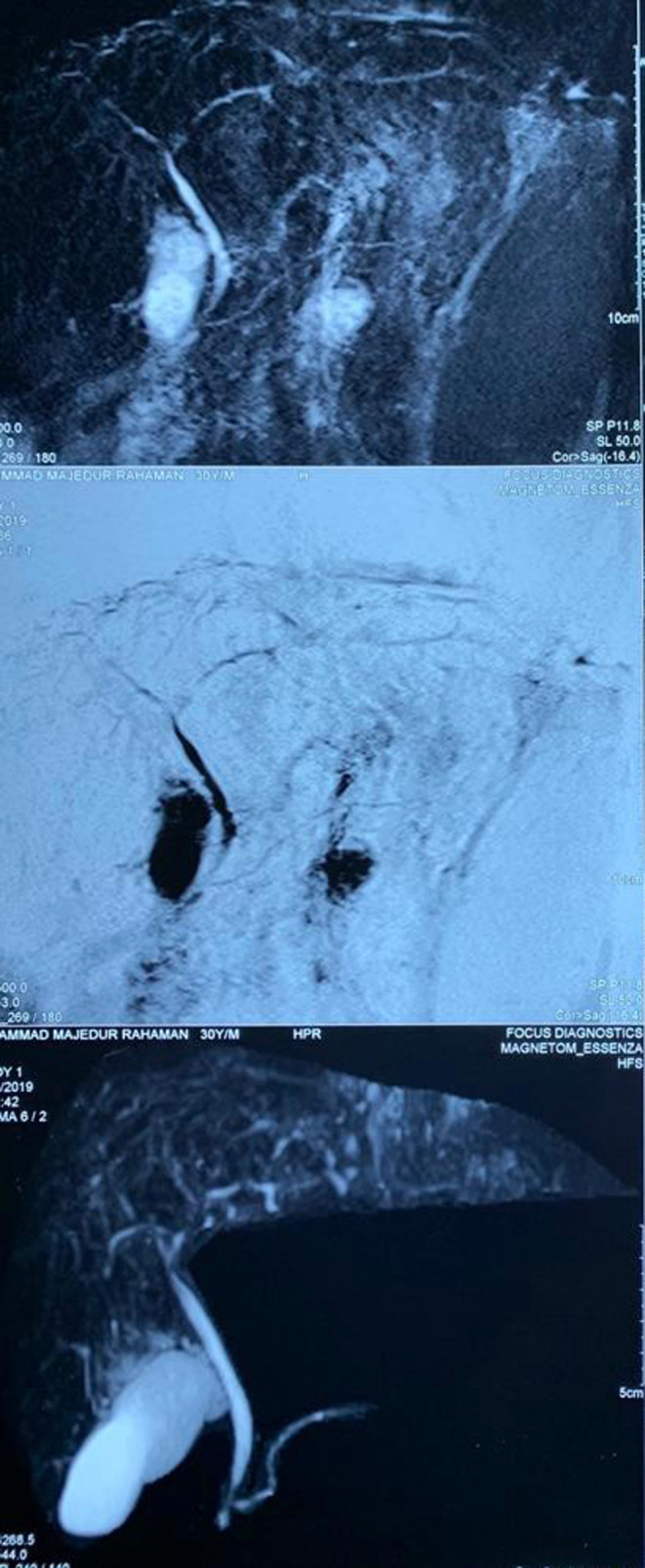
Magnetic resonance cholangiopancreatography.
In light of the patient's cirrhosis accompanied by elevated ALP levels and lymphadenopathy and after ruling out common causes of cirrhosis, we conducted a serum angiotensin‐converting enzyme (ACE) test to explore the possibility of sarcoidosis, which revealed elevated levels.
Despite the absence of cough or respiratory distress, we conducted a high‐resolution chest CT (HRCT) to further investigate the suspicion of sarcoidosis. HRCT revealed multiple areas of peripheral ground‐glass opacities in the right middle and bilateral upper lobes, as well as a few areas of reticular thickening in the bilateral lower lobes. No enlarged mediastinal lymph nodes were observed (Figure 3). Spirometry was normal, but reduced diffusion of carbon monoxide in the lungs was detected, and the 6‐minute walk test showed impaired lung function.
FIGURE 3.
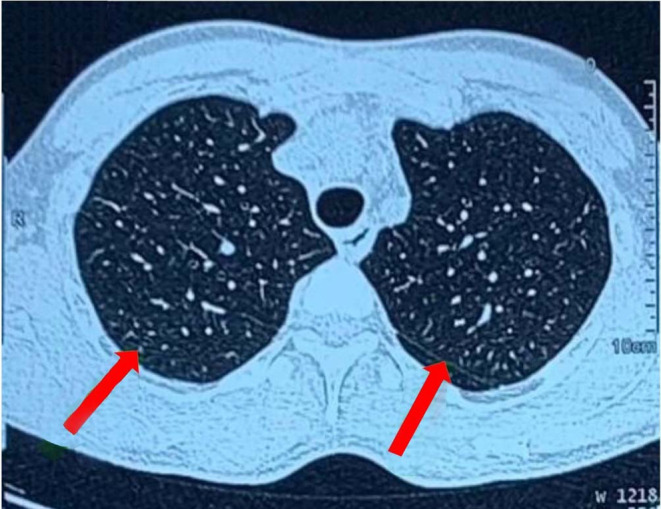
High‐resolution chest computed tomography showing peripheral ground glass opacity.
The patient was counseled for a liver biopsy, and he consented. Histopathological examination revealed noncaseating granulomatous inflammation with fibrotic areas (Figure 4). Staining for acid‐fast bacilli, cytology, and gene experts in the bronchoalveolar lavage yielded negative results. Based on these findings, the patient was diagnosed with decompensated cirrhosis of the liver secondary to sarcoidosis.
FIGURE 4.
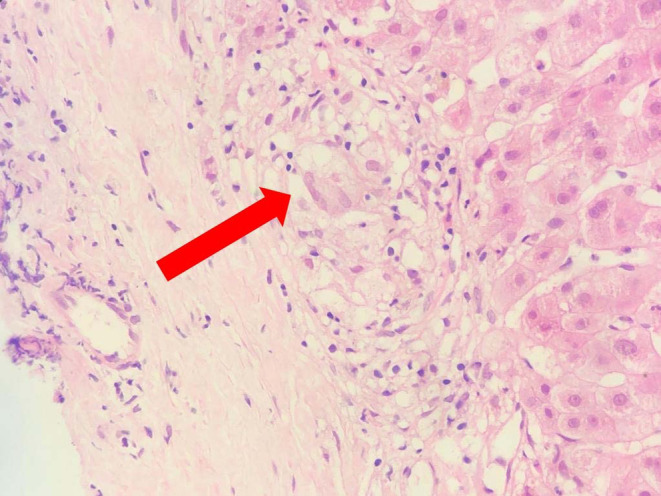
Liver histopathology showing noncaseating granulomatous inflammation.
The patient was started on prednisolone at 40 mg/day and discharged. The possible side effects of steroids were explained to the patient prior to discharge.
4. FOLLOW‐UP
After 3 months of prednisolone treatment, there was a slight improvement in his liver enzymes. However, once cirrhosis develops, the likelihood of liver recovery is low. (Table 1).
TABLE 1.
Liver function test on admission and 3 months after starting steroid.
| LFT | Admission | Follow‐up at 3 months | Reference value |
|---|---|---|---|
| S. bilirubin | 22.23 μmol/L | 20.52 μmol/L | 5.1–20.5 μmol/L |
| AST | 80 U/L | 53 U/L | 10–34 U/L |
| ALT | 84 U/L | 57 U/L | 10–49 U/L |
| ALP | 240 U/L | 120 U/L | 46–116 U/L |
| GGT | 148 U/L | 85 U/L | <38 U/L |
| S. albumin | 34 gm/L | 35 gm/L | 35–50 gm/L |
| Prothrombin time | 12 s | 12 s | 12 s |
5. DISCUSSION
Sarcoidosis is a systemic disease characterized by the formation of noncaseating granulomas in various organs. Its precise pathophysiology remains unclear but is believed to result from an exaggerated immune response triggered by an unknown antigen in genetically predisposed individuals. 6 It can affect multiple organs in the body; however, lung involvement is the most common (90% of cases). Although liver involvement is not uncommon in sarcoidosis, most cases are asymptomatic with only granulomatous inflammation in the liver. 6 However, asymptomatic liver enzyme elevation occurs in half of hepatic sarcoidosis cases. Symptomatic liver involvement has been observed in approximately 15% of patients. Common symptoms may include abdominal pain and itching. 3 , 7
In rare cases, sarcoidosis can lead to cirrhosis. Cirrhosis can occur as a result of extensive granuloma formation, causing fibrosis or secondary biliary cirrhosis due to bile duct inflammation. 6 , 8 Although sarcoidosis has been reported to cause cirrhosis in some cases, the initial symptoms are usually related to lung involvement. 9 , 10 However, our patient presented with no respiratory symptoms, making the diagnosis of sarcoidosis‐associated cirrhosis difficult. This underscores the importance of considering sarcoidosis as a potential underlying condition in patients presenting with cirrhosis of unknown etiology even in the absence of typical respiratory symptoms.
In sarcoidosis, ALP and GGT levels are typically higher than aminotransferase levels. 3 Granulomatous bile duct inflammation can mimic PSC. In some reported cases, patients were initially misdiagnosed with PSC. 11 , 12 Our patient was initially suspected of having PSC owing to his clinical presentation, but a negative history of inflammatory bowel disease and negative p‐ANCA test results prompted the physician to perform a liver biopsy.
Serum ACE levels were elevated in 75% of the sarcoidosis. 3 Our patient also showed elevated serum ACE levels. Although other conditions may also cause elevated ACE levels, further investigation is necessary to determine whether sarcoidosis is the underlying cause.
Although there is no established treatment protocol for sarcoidosis‐associated liver involvement, steroids are widely recognized as the primary treatment for cases involving the lungs, eyes, and central nervous system. 13 Although asymptomatic liver involvement may not require treatment, symptomatic liver disease should be treated. Treatment may lead to the improvement of symptoms and liver function tests in cases of mild to moderate abnormalities. However, improvement is less likely in severe cases of sarcoidosis‐associated liver disease. 13 , 14 During the short follow‐up period, our patient demonstrated good improvements in symptoms but biochemical markers were improved slightly. However, cirrhosis is an irreversible condition, and although treatment may slow disease progression, it cannot reverse it.
AUTHOR CONTRIBUTIONS
- Case Involvement and Contribution:
- Sumona Islam: Contributes to the diagnosis, treatment and follow‐up of the case.
- Md Intisar Kamal: Contributes to data collection and interpretation related to the case.
- Khaled Murshed: Detail involvement in literature review, case background research, or specific medical aspects.
- Md Abul Kalam Azad: Contributes to the discussion and implications of the case report.
- Drafting or Critical Revision:
- Sumona Islam: Involves in drafting the case report and significant revisions.
- Md Intisar Kamal: Contributes to the critical revision and drafting process.
- Khaled Murshed: Contributes to subsequent critical revisions.
- Md Abul Kalam Azad: Contributes to critical review stages.
- Final Approval:
- All authors have reviewed and given final approval for the version of the case report to be submitted for publication.
- Accountability and Investigation:
- All authors collectively agree to be accountable for the accuracy and integrity of the case report.
- Commit to addressing any concerns or queries about the case report's content or data.
FUNDING INFORMATION
No funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We thank Editage (www.editage.com) for English language editing.
Islam S, Kamal I, Murshed K, Azad MAK. Cirrhosis due to hepatic sarcoidosis: A rare presentation. Clin Case Rep. 2024;12:e8999. doi: 10.1002/ccr3.8999
DATA AVAILABILITY STATEMENT
All the data related to this case report are available to corresponding author and will be available upon request.
REFERENCES
- 1. Hena KM. Sarcoidosis epidemiology: race matters. Front Immunol. 2020;11:537382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the black Women's health study. Chest. 2011;139(1):144‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sedki M, Fonseca N, Santiago P, et al. Hepatic sarcoidosis: natural history and management implications. Front Med (Lausanne). 2019;6:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885‐1889. [DOI] [PubMed] [Google Scholar]
- 5. Blich M, Edoute Y. Clinical manifestations of sarcoid liver disease. J Gastroenterol Hepatol. 2004;19(7):732‐737. [DOI] [PubMed] [Google Scholar]
- 6. Tadros M, Forouhar F, Wu GY. Hepatic sarcoidosis. J Clin Transl Hepatol. 2013;1(2):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryland KL. Hepatic sarcoidosis: incidence, monitoring, and treatment. Clin Liver Dis (Hoboken). 2020;16(5):208‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Judson MA. Hepatic, splenic, and gastrointestinal involvement with sarcoidosis. Semin Respir Crit Care Med. 2002;23(6):529‐541. [DOI] [PubMed] [Google Scholar]
- 9. Tan CB, Rashid S, Rajan D, Gebre W, Mustacchia P. Hepatic sarcoidosis presenting as portal hypertension and liver cirrhosis: case report and review of the literature. Case Rep Gastroenterol. 2012;6(1):183‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fauter M, Rossi G, Drissi‐Bakhkhat A, et al. Hepatic sarcoidosis with symptomatic portal hypertension: a report of 12 cases with review of the literature. Front Med (Lausanne). 2022;9:995042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gavilán F, Pereda T, Sousa JM, et al. Hepatic cirrhosis with sarcoid granulomas. Differential diagnosis and liver transplantation: a case report. Transplant Proc. 2003;35(2):713‐714. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Ciobanu C, Mohrmann L. Hepatic sarcoidosis resembling primary sclerosing cholangitis. BMJ Case Rep. 2021;14(9):e243492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184‐3192; quiz 93. [DOI] [PubMed] [Google Scholar]
- 14. Vatti R, Sharma OP. Course of asymptomatic liver involvement in sarcoidosis: role of therapy in selected cases. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(1):73‐76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data related to this case report are available to corresponding author and will be available upon request.


