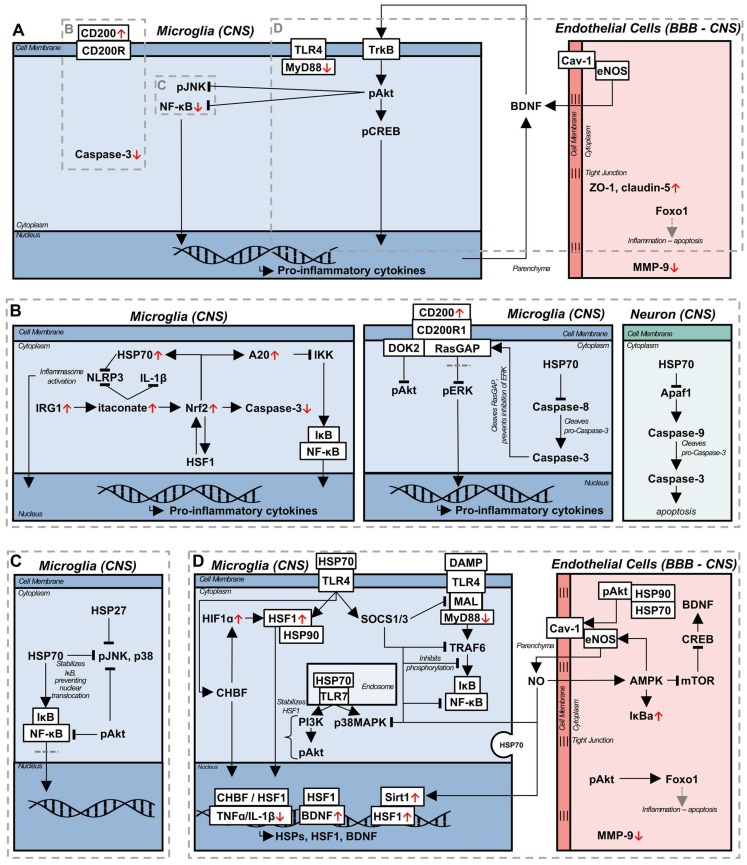
Figure 3.
Comparison of the observed anti-inflammatory effects of LIPUS, with proposed HSR induced anti-inflammatory signalling pathways as an explanatory mechanism. (A) Experimentally observed changes in key signalling pathways proposed as the mechanisms behind LIPUS mediated resolution of inflammation. (B) Signalling pathways upregulated during the HSR, and mild hyperthermia, capable of inhibiting inflammasome formation, and downregulating caspase-3 activation (left). Under inflammatory conditions, CD200/CD200R signalling can become dysregulated, with caspase-3 cleaving RasGAP, leading to the differential inhibition of pAkt, while losing the ability to inhibit ERK activation. Microglial activation is dependent on the caspase-8/caspase-3 pathway, allowing HSP70 to both inhibit activation, and restore homeostatic CD200/CD200R signalling (middle). HSP70 also inhibits the neuronal apoptosis pathway (right). (C) In addition to the proposed LIPUS mediated inhibition of JNK phosphorylation, and NF-kB activation, through pAkt, HSP27 and HSP70 inhibit pJNK, while HSP70 additionally prevents p38 MAPK phosphorylation, and IKK mediated degradation of IkB. (D) HSP70 has been described to bind to TLR4, upregulating SOCS1/3 which directly inhibits MAL and subsequent MyD88 TLR4 signalling complex formation, and pro-inflammatory cytokine production. Additionally, HSP70 and TLR4 interactions are associated with increased HSF1, and CHBF transcription, both of which are capable of binding to the heat shock elements of target genes, downregulating pro-inflammatory cytokines, while upregulating neurotrophic factors. Crosstalk between stress response pathways, such as the hypoxia and heat stress responses, generates a compensatory, symbiotic cascade of anti-inflammatory signalling pathways, with CHBF, HIF1a, and HSF1 facilitating mutual gene expression. TLR7 and HSP70 have been shown to interact, and activate the PI3k/pAkt pathway, which is implicated in stabilizing HSF1 and upregulating the HSR. Through extracellular vesicles, and direct protein secretion, adjacent cells can communicate and influence the cellular environment, and activation states. In studies of LIPUS, eNOS was characterized as an essential component for an anti-inflammatory effect, and was correlated with the upregulation of BDNF, while a mechanism was not proposed. Pharmaceutical upregulation of eNOS is predicated on a complex of phosphorylated Akt, and HSP90, with potential co-chaperone behaviour of HSP70, eventually leading to NO release. NO stimulates the AMPK/mTOR/CREB/BDNF pathway, potentially explaining the observed relationship between eNOS and BDNF release. Simultaneously, NO upregulates Sirt1, a transcriptional regulator necessary for sufficient induction of the HSR.