Figure 3.
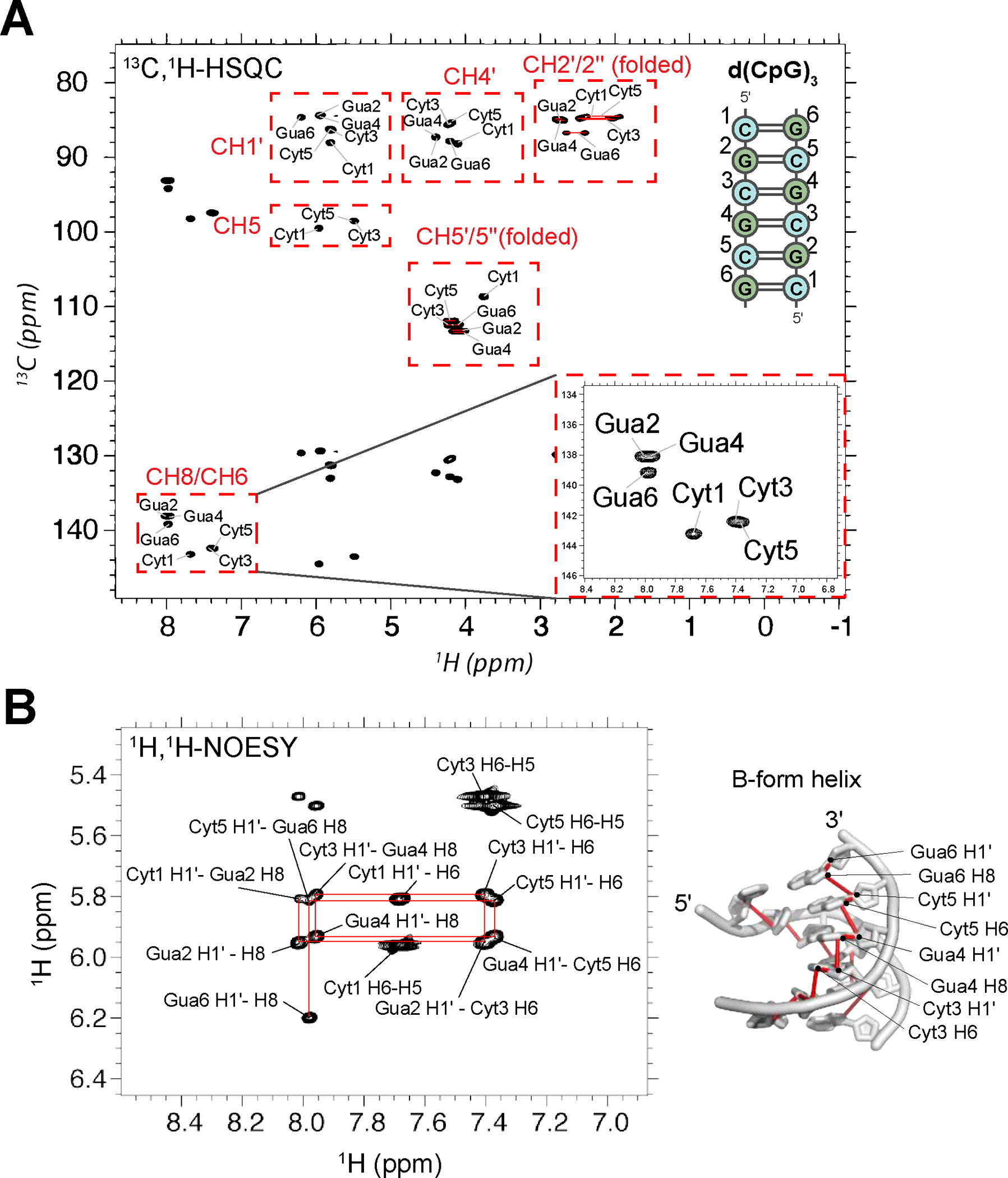
NMR assignment of the d(CpG)3 construct. (a) Full 13C-1H HSQC spectra assignments for the d(CpG)3 construct are shown (depicted on the right with assignment numbering). The CH2’/2” and CH5’/5” peak positions are folded in from their normal positions around 40 and 66 ppm, respectively. Their proper chemical shift values are indicated in Table S1. CH3’ resonances were not assignable due to water suppression. Inset shows a zoom in of the aromatic assignments. Note that the two strands of the duplex are chemically equivalent and, therefore, have identical chemical shifts. (b) 1H-1H NOESY experiment with a mixing time of 320 ms showing the aromatic H8/H6 to ribose H1′ connectivites. The NOESY “walk” through the B-form helix is indicated with red lines, an example of which is shown on the structure of a B-form helix (PDB: 1N1K1) to the right.
