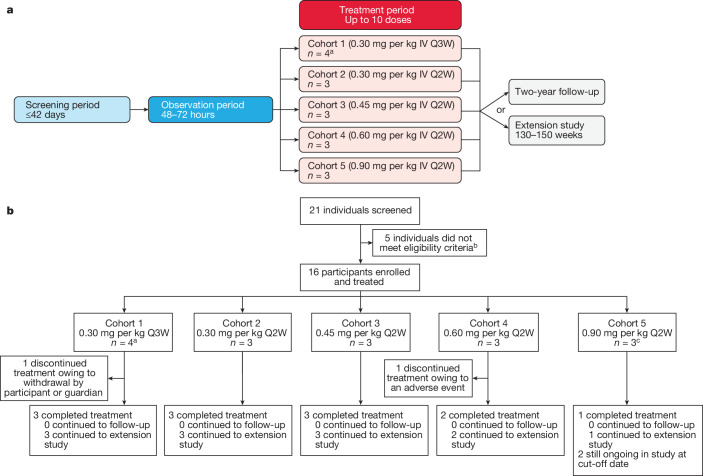
Fig. 2. Dose-optimization study design and participant information.
a, Study schema. Participant eligibility was assessed during the screening period, followed by an observation period during which baseline PA disease status was monitored. The enrolment of participants into each cohort was staggered using a sentinel dosing strategy. Three participants were enrolled into a cohort, treated and monitored for safety for at least 14 days by a Safety Monitoring Committee. After confirmation that no DLTs occurred, enrolment and dosing of the next cohort began. After the treatment period, participants could roll over into the extension study or enter a two-year follow-up period. Q2W, every two weeks; Q3W, every three weeks. b, CONSORT diagram. aAn additional participant was enrolled in cohort 1 per protocol after the discontinuation of one participant after their first dose owing to the withdrawal of participant consent. bSeven individuals failed the initial screening; two of these individuals were rescreened and met eligibility criteria for inclusion in the study. cOne participant switched to 0.60 mg per kg every two weeks dosing.