Abstract
The aim of this study was to determine whether caffeine enhanced radiosensitization in an orthotopic transplant of LM3 human hepatocellular cancer in nude mice. LM3 hepatocellular carcinoma cells were infected with red fluorescent protein and irradiated, and cell cycle distribution and survival fraction were detected. A nude mouse model of orthotopic transplant of red fluorescent protein‐expressing LM3 hepatocellular cancer was established. Nude mice were divided into four groups: control (NS); caffeine (Caff) alone; irradiation (IR) alone; and caffeine + IR (Caff + IR). Tumor growth curves were described. Expression of cyclin and apoptosis were evaluated by analysis of phosphorylated cyclin dependent kinase 1 (CDC2) Tyr15 (CDC2‐Tyr15‐P), cyclinB1, TUNEL staining, and caspase‐3. Caffeine abrogated IR‐induced G2 phase arrest and decreased survival of irradiated LM3 cells. Caffeine enhanced radiosensitivity of LM3 hepatocellular cancer in vivo. Tumor growth delay time in the Caff + IR group was 14.3 days compared with the NS group, 14.1 days compared with the Caff alone group, and 7.2 days compared with the IR alone group. At 15 Gy, expression of CDC2‐Tyr15‐P in the Caff + IR group (26.0 ± 8.9%) was significantly lower than in the IR alone group (68.4 ± 10.6%), expression of cyclinB1 and proportion of TUNEL‐positive cells in the Caff + IR group (30.4 ± 8.7% and 59.2 ± 9.5%, respectively) was significantly higher than in the IR alone group (7.0 ± 3.7% and 24.2 ± 7.2%, respectively), expression of caspase‐3 was consistent with the TUNEL staining results. This study suggested that caffeine might enhance the radiosensitivity of LM3 hepatocellular cancer in vivo, and may be feasible for further clinical applications. (Cancer Sci 2010)
Hepatocellular cancer is the sixth most common cancer and the third most common cause of cancer‐related death worldwide because of its poor prognosis.( 1 ) Surgical resection is the best choice for treatment of hepatocellular cancer. However, a considerable number of cases are not suitable for surgical resection because of factors such as the large size of the tumor mass, and the coexistence of liver cirrhosis and portal vein tumor thrombosis. Radiotherapy is an effective treatment option for inoperable hepatocellular cancer,( 2 , 3 ) although irradiation (IR)‐induced liver disease limits the dose that can be delivered.
Caffeine, a well‐known radiosensitizing agent, is believed to increase radiosensitivity by enhancing apoptosis.( 4 , 5 , 6 ) In response to various DNA damaging agents such as IR, cells undergo G2 delay, which provides sufficient time for DNA repair to maintain genomic integrity.( 7 ) The ability of caffeine to increase the radiosensitivity of cells is due to the inhibition of G2 checkpoint activation.( 8 , 9 , 10 ) Radiation‐induced G2 phase arrest is closely related to the inhibition of cyclin dependent kinase 1 (CDC2) activity,( 11 , 12 ) and activation of CDC2 in combination with cyclinB1 is an important condition for G2/M transition in the cell cycle.( 13 ) Suppression of CDC2 activity contributes to phosphorylation of CDC2 Tyr15 protein after IR.( 14 , 15 ) Caffeine interferes with phosphorylation of CDC2 Tyr15 protein, resulting in increased CDC2 activity,( 16 ) which is necessary to promote the cell to enter the M phase. Radiation might directly reduce the expression of cyclinB1,( 17 , 18 ) whereas caffeine can increase the expression of cyclinB1 after IR.( 19 ) Therefore, caffeine might enhance radiosensitivity by increasing the activity of the CDC2/cyclinB1 complex, which decreases the time available for damaged DNA to undergo repair, and hence increases the IR‐induced aberration rate of DNA, ultimately resulting in apoptosis.( 20 )
Caffeine has been extensively tested as a radiosensitizer in studies in vitro. However, to our knowledge, the radiosensitization effect of caffeine in vivo is unclear, because achieving millimolar concentrations required for the effective dose of caffeine in vitro is difficult. Nevertheless, caffeine is almost 100% bioavailable when taken orally,( 21 ) it is absorbed by the stomach and small intestine promptly after ingestion, accumulates rapidly in the portal vein, and reaches the liver, where it is concentrated in liver tumor tissue. As a result, we hypothesized that caffeine could be uniquely suited to enhance hepatocellular cancer radiosensitization.
In the present study, we used a nude mouse model with orthotopic transplant of hepatocellular cancer with IR exposure of 5 Gy per fraction delivered every other day, to investigate whether caffeine increases the radiosensitivity of LM3 hepatocellular cancer and by what possible mechanism, and to determine the feasibility of further clinical application.
Materials and Methods
Cell fluorescent protein infection, culture, and drug treatment. LM3 hepatocellular carcinoma cell lines, with high metastatic potential and mutant p53, were established and provided by the Liver Cancer Institute of Fudan University (Shanghai, China).( 22 ) The cells were infected with red fluorescent protein (RFP) full‐length cDNA by a lentivirus as described previously( 23 ) and cultured in high glucose DMEM supplemented with 10% FBS (Gibco‐BRL, Grand Island, NY, USA) at 37°C in a humidified incubator in an atmosphere of 5% CO2. Caffeine (Sigma, St. Louis, MO, USA) was dissolved in the medium at 2.5 mM and used for incubation of LM3 cells immediately after IR.
Flow cytometry analysis. Exponentially growing anchorage‐dependent LM3 cells were irradiated using a 6‐MV X‐ray beam (Oncor; Siemens, Erlangen, Germany), and fixed in 75% ethanol at −4°C overnight in the corresponding time after IR. Cells were suspended in 1 mL PBS containing 50 μg RNase‐A and 65 μg propidium iodide at 37°C for 1 h in the dark. Cell cycle distribution was determined using a flow cytometer (FACSCalibur; Becton Dickinson Biosciences, San Jose, CA, USA).
Clonogenic cell survival assay. LM3 cells were plated in 6‐well plates, incubated overnight, and irradiated using an accelerator (Oncor; Siemens). The cells were further incubated with caffeine for 72 h and cultured in fresh medium for 14 days. The colonies were fixed with methanol and stained with 1% crystal violet. Only the colonies containing more than 50 cells were counted, and survival fractions were calculated.
Establishing the orthotopic transplant hepatocellular cancer model and irradiation. Four‐week‐old male BALB/c hairless mice were purchased from SiLaike Animal Company (Shanghai, China). The treatment of hairless mice and this study were approved by the local animal ethics committee. The nude mice were s.c. injected with 1 × 107 RFP‐expressing LM3 cells into the upper flank region. When the tumors reached 1 cm in diameter, they were removed, cut into 2 × 2 × 2 mm3‐sized pieces, and implanted into the liver, as described previously,( 24 ) to establish an RFP‐expressing LM3 hepatocellular cancer orthotopic xenograft model. The mice were maintained under pathogen‐free conditions with access to a normal laboratory diet and water ad libitum after surgery. The mice were randomly divided into four groups: control (normal saline [NS]); caffeine (Caff) alone; IR alone; and caffeine + IR (Caff + IR). When the intrahepatic tumor fluorescent area, which generally represents the tumor tissue size, reached approximately 0.5 cm in diameter under fluorescence microscopy, the margins of tumor areas were marked on the skin of each animal. The intrahepatic tumors in both IR groups were exposed to IR of 5 Gy per fraction for three fractions, every other day, for a total of 15 Gy, using a 6‐MeV electronic beam at a dose rate of 300 cGy per minute (Oncor; Siemens), while normal tissue was protected using lead shielding. Caffeine in solution (2 mg per 1 mL) at a dose of 100 mg/kg was injected into the stomachs of mice in both caffeine groups using gavage immediately after completion of IR, with the same volume of saline given to mice in the control and IR alone groups. The red fluorescent areas were imaged by using fluorescence microscopy before IR and every 3 days after IR. Image‐Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used to measure the size of the red fluorescent areas, and the resulting tumor growth curves were described. All animals were killed 30 days after IR. Tumor tissues were isolated, and length (a), width (b), and height (c) of the tumors were measured using calipers. Tumor volume was calculated using the formula V = (4π/3) × (a/2) × (b/2) × (c/2), and a tumor volume histogram was described.
Western blot analysis. Tumor tissues were obtained at 12 h and 36 h after 5‐Gy IR and 12 h after 10‐Gy and 15‐Gy IR. Tumor tissues were lysed in solubilizing buffer (1% Triton X‐100, 1% deoxycholate, 0.1% SDS, 1 mmol/L PMSF, and a protease inhibitor cocktail) and centrifuged. The samples were separated by 10% SDS‐PAGE and transferred onto PVDF membranes. The membranes were incubated with the main antibody, either CDC2‐Tyr15‐P, cyclinB1, or caspase‐3 (Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C, and HRP‐conjugated rabbit antimouse antibody (Dako, Copenhagen, Denmark) was applied. The immunoreactive signal was visualized with an ECL detection system (Amersham Pharmacia Biotech, Buckinghamshire, UK). The optical density value of each protein was normalized to the tubulin level in the same sample. Each experiment was repeated five times.
Immunohistochemical analysis and TUNEL assay. Tumor tissues were obtained 12 h after completion of 5‐Gy, 10‐Gy, or 15‐Gy IR. Tumor tissues were fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin. After dewaxing, endogenous peroxidase was blocked using 3% H2O2 for 30 min, and samples were incubated with main antibodies to CDC2‐Tyr15‐P and cyclinB1 (Cell Signaling Technology) overnight at 4°C in a humidity chamber, and HRP‐conjugated rabbit antimouse antibody (Dako) and 3,3‐diaminobenzidine were applied. For TUNEL staining, deparaffinized sections were incubated with terminal deoxynucleotidyl transferase and biotin‐16‐dUTP (Roche Applied Science, Indianapolis, IN, USA), signals were visualized after adding HRP‐conjugated antibiotin antibody and 3,3‐diaminobenzidine. Ten random high‐power fields were examined, and the number of positive cells was manually counted by two physicians. The proportion of positive cells was calculated as the number of positive cells divided by the total number of cells ×100%.
Statistical analysis. Data were expressed as the mean ± SD. Comparison among multiple groups was carried out with a non‐parametric anova test. Results with P < 0.05 were considered statistically significant.
Results
Radiation‐induced G2 phase arrest was abolished by caffeine in LM3 cells. The proportion of LM3 cells in G2 phase arrest 16 h after IR increased with increasing IR dose, with 13.7 ± 2.9% at 0 Gy, 25.9 ± 3.6% at 8 Gy, 40.8 ± 3.3% at 16 Gy, and 52.2 ± 4.4% at 24 Gy. In contrast, IR did not induce G1 phase arrest (Fig. 1A). The proportion of cells in G2 phase arrest reached a maximum of 39.9 ± 2.2% at 12 h after 12 Gy IR, whereas the proportion of cells in G2 phase arrest treated with 2.5 mM caffeine and 12‐Gy IR was 21.7 ± 2.3% (Fig. 1B). The proportion of cells treated with 2.5 mM caffeine without IR in G2 phase arrest was similar to the controls at the corresponding time (data not shown).
Figure 1.
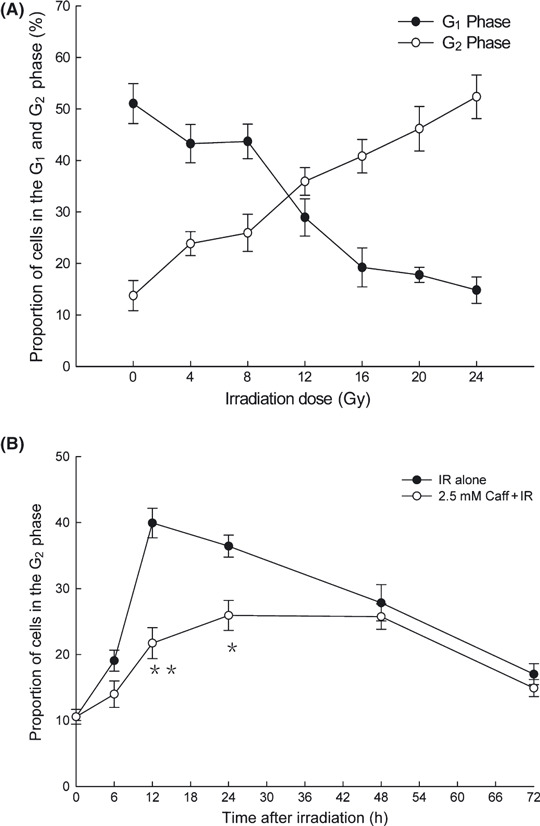
Caffeine abolished radiation‐induced G2 phase arrest in LM3 hepatocellular carcinoma cells. (A) The proportion of cells in G1 phase arrest and G2 phase arrest as a function of dose 16 h after irradiation (IR). (B) The proportion of cells in G2 phase arrest as a function of time after 12 Gy IR with or without caffeine (Caff). Data are expressed as the mean ± SD for five independent experiments. *P < 0.05 compared with the IR alone group; **P < 0.01 compared with the IR alone group.
Caffeine sensitizes cells to ionizing radiation. As shown in Figure 2, the survival of LM3 cells decreased with IR alone in a dose‐dependent manner. Adding caffeine decreased survival even further, also dependent on the IR dose. Survival of cells treated with 2.5 mM caffeine alone was similar to the control group (data not shown).
Figure 2.
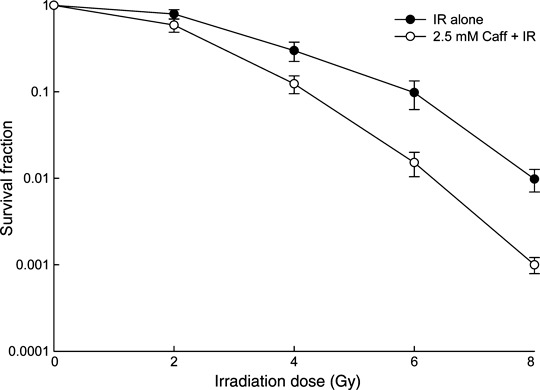
Clonogenic surviving fraction of LM3 hepatocellular carcinoma cells following treatment with irradiation (IR) alone or IR with 2.5 mM caffeine (Caff). The surviving fraction of cells was normalized by the occurrence of cell death in the group treated with 0 Gy IR. Data are expressed as the mean ± SD for five independent experiments.
Caffeine sensitizes tumor tissues to ionizing radiation. As shown in Figure 3(A,B), the tumor fluorescent areas in each group before IR did not differ significantly; however, the tumor fluorescent area in the Caff+IR group was significantly smaller than that of the control group (P = 0.014) on day 6 from the beginning of IR, of the Caff alone group (P = 0.026) on day 9, and of the IR alone group (P = 0.043) on day 12. The differences increased as time went on. The tumor fluorescent area in the Caff alone group and the NS group did not differ significantly. The tumor fluorescent area increased to four times larger than the initial area in the control group in 14.7 days, in the Caff alone group in 14.9 days, in the IR alone group in 21.8 days, and in the Caff + IR group in 29.0 days. The tumor growth delay time in the Caff + IR group was 14.3 days compared with the control group, 14.1 days compared with the Caff alone group, and 7.2 days compared with the IR alone group, whereas the tumor doubling time between the control and Caff alone groups showed almost no difference. As shown in Figure 3(C), tumor volume at the end of experiment in the Caff + IR group was significantly smaller than the IR alone group.
Figure 3.
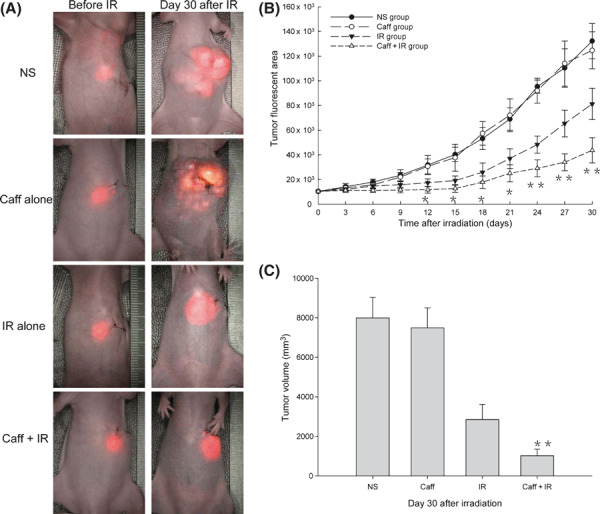
Caffeine (Caff) sensitized hepatocellular tumor tissues to ionizing irradiation (IR). (A) Tumor fluorescence images before IR and at day 30 after IR. (B) Tumor growth curves. (C) Tumor volume histogram at the end of the experiment. Data are expressed as the mean ± SD (n = 10 per group). NS, normal saline. *P < 0.05 compared with the IR alone group; **P < 0.01 compared with the IR alone group.
Caffeine attenuated CDC2‐Tyr15‐P protein expression to ionizing radiation. The results of Western blot investigation of CDC2‐Tyr15‐P protein expression are presented in Figure 4(A). There were no significant differences in CDC2‐Tyr15‐P protein expression between the NS and Caff groups. Levels of CDC2‐Tyr15‐P protein 12 h after IR increased with increasing IR dose in the IR alone group. The CDC2‐Tyr15‐P protein expression level was much lower in the Caff + IR group compared with the IR alone group at the same IR dose. However, the protein expression level of CDC2‐Tyr15‐P in the Caff + IR group was similar to the level in the IR alone group, with both at a high level 36 h after 5‐Gy IR.
Figure 4.
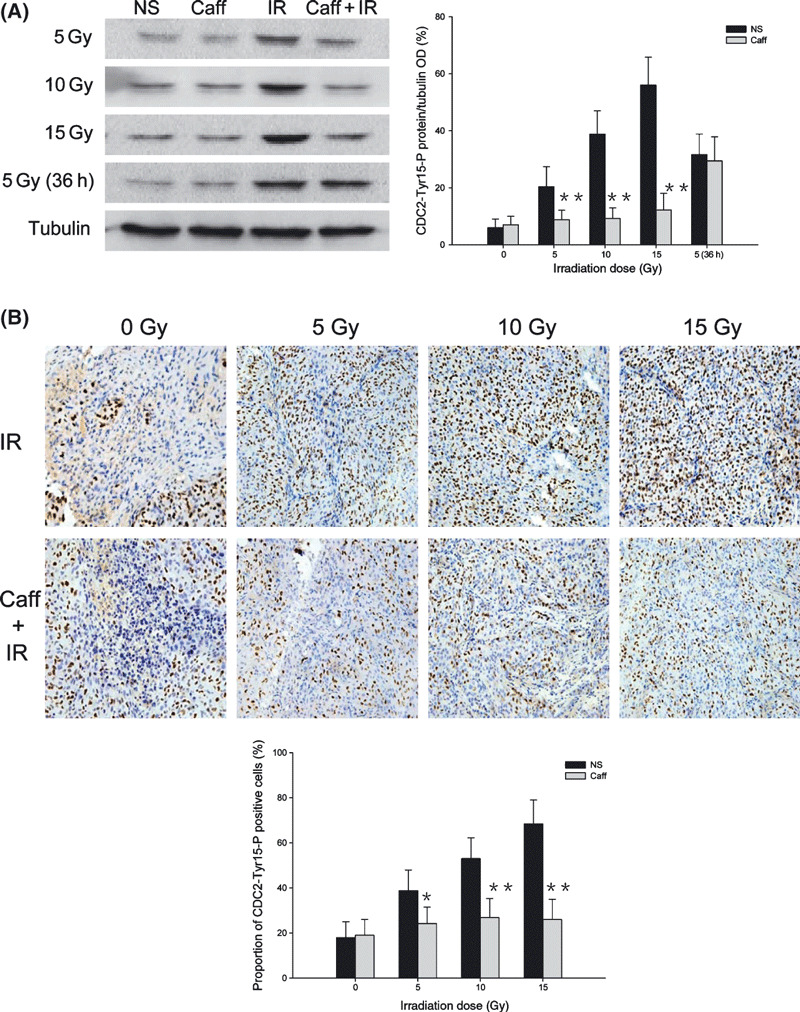
Phosphorylated cyclin dependent kinase 1 (CDC2) Tyr15 (CDC2‐Tyr15‐P) protein expression in LM3 hepatocellular carcinoma cells and the effect of irradiation (IR) with or without caffeine (Caff). (A) Western blot analysis of CDC2‐Tyr15‐P expression at 0 Gy, 12 h and 36 h after 5‐Gy IR, and 12 h after 10‐Gy and 15‐Gy IR. (B) Immunohistochemical analysis of CDC2‐Tyr15‐P positive cells at 0 Gy, 12 h after 5‐Gy, 10‐Gy, and 15‐Gy IR. The number of positive cells in 10 randomly selected areas was counted under the microscope (original magnification, ×200). Data are expressed as the mean ± SD (n = 5 per group). NS, normal saline. *P < 0.05 compared with the IR alone group; **P < 0.01 compared with the IR alone group.
The results of quantitative immunohistochemical analysis for CDC2‐Tyr15‐P for different IR doses, presented in Figure 4(B), were consistent with Western blot results. In the NS group and Caff group, the proportion of CDC2‐Tyr15‐P positive cells were 18.9 ± 7.1% and 19.6 ± 7.4%, respectively. In the IR alone group, the proportion of CDC2‐Tyr15‐P positive cells with 5‐Gy IR was 38.8 ± 9.2%; with 10‐Gy IR, 53.0 ± 9.2%; and with 15‐Gy IR, 68.4 ± 10.6%. In the Caff + IR group, the proportions of CDC2‐Tyr15‐P positive cells were significantly lower than in the IR alone group; with 5‐Gy IR, the proportion of positive cells was 24.2 ± 7.3%; with 10‐Gy IR, 26.8 ± 8.5%; and with 15‐Gy IR, 26.0 ± 8.9%.
Caffeine enhanced cyclinB1 protein expression to ionizing radiation. The results of Western blot investigation of cyclinB1 protein expression are presented in Figure 5(A). There were no significant differences in cyclinB1 protein expression between the NS and Caff groups. In the IR alone group, expression of cyclinB1 protein 12 h after IR decreased with increasing IR dose. In the Caff + IR group, expression of cyclinB1 protein was much higher than that of the IR alone group at the same IR dose. Similar to CDC2‐Tyr15‐P protein expression, cyclinB1 protein expression did not differ between the Caff + IR group and the IR alone group, with both at a low level 36 h after 5‐Gy IR.
Figure 5.
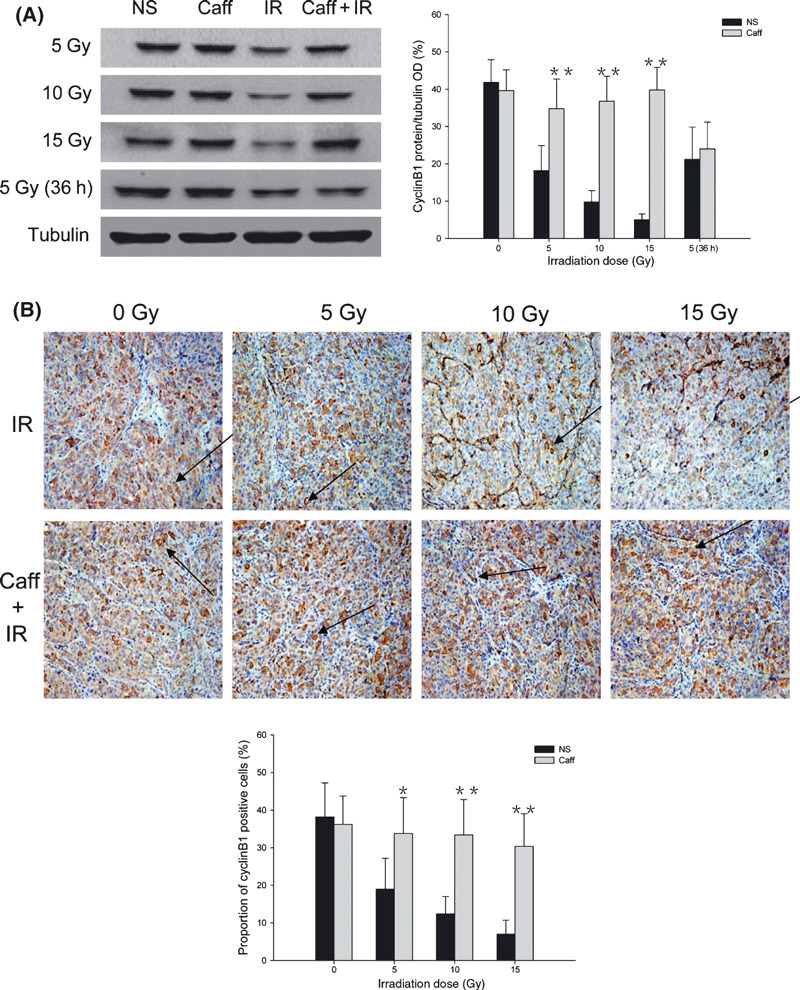
CyclinB1 protein expression in LM3 hepato‐cellular carcinoma cells and the effect of irradiation (IR) with or without caffeine (Caff). (A) Western blot analysis of cyclinB1 expression at 0 Gy, 12 h and 36 h after 5‐Gy IR, and 12 h after 10‐Gy and 15‐Gy IR. (B) Immunohistochemical analysis of cyclinB1‐positive cells at 0 Gy, 12 h after 5‐Gy, 10‐Gy, and 15‐Gy IR. The number of positive cells in 10 randomly selected areas were counted under the microscope (original magnification, ×200). Arrows indicate cyclinB1‐positive cells. Data are expressed as the mean ± SD (n = 5 per group). NS, normal saline. *P < 0.05 compared with the IR alone group; **P < 0.01 compared with the IR alone group.
The results of quantitative immunohistochemical analysis for cyclinB1 for different IR doses, presented in Figure 5(B), were also consistent with Western blot results. In the NS and Caff groups, the proportion of cyclinB1‐positive cells were 38.2 ± 9.0% and 36.2 ± 7.6%, respectively. In the IR alone group, the proportion of cyclinB1‐positive cells with 5‐Gy IR was 19.0 ± 8.2%; with 10‐Gy IR, 12.4 ± 4.6%; and with 15‐Gy IR, 7.0 ± 3.7%. In contrast, in the Caff + IR group, the proportions of cyclinB1‐positive cells were significantly higher than in the IR alone group; with 5‐Gy IR, the proportion of cyclinB1‐positive cells was 33.8 ± 9.5%; with 10‐Gy IR, 33.4 ± 9.3%; and with 15‐Gy IR, 30.4 ± 8.7%.
Caffeine enhanced apoptosis to ionizing radiation. Staining results from TUNEL assay are presented in Figure 6(A). In the NS and Caff groups, the proportion of TUNEL‐positive nuclei were 14.4 ± 8.2% and 15.2 ± 8.8%, respectively. In the IR alone group, the proportion of TUNEL‐positive nuclei was 18.6 ± 8.2% with 5‐Gy IR; 21.2 ± 6.1% with 10‐Gy IR; and 24.2 ± 7.2% with 15‐Gy IR, with no significant difference between the different IR doses. In the Caff + IR group, the proportions of TUNEL‐positive nuclei were significantly higher than in the IR alone group. With 5‐Gy IR, the proportion of TUNEL‐positive nuclei was 31.0 ± 8.8%; with 10‐Gy IR, 43.2 ± 10.9%; and with 15‐Gy IR, 59.2 ± 9.5%.
Figure 6.
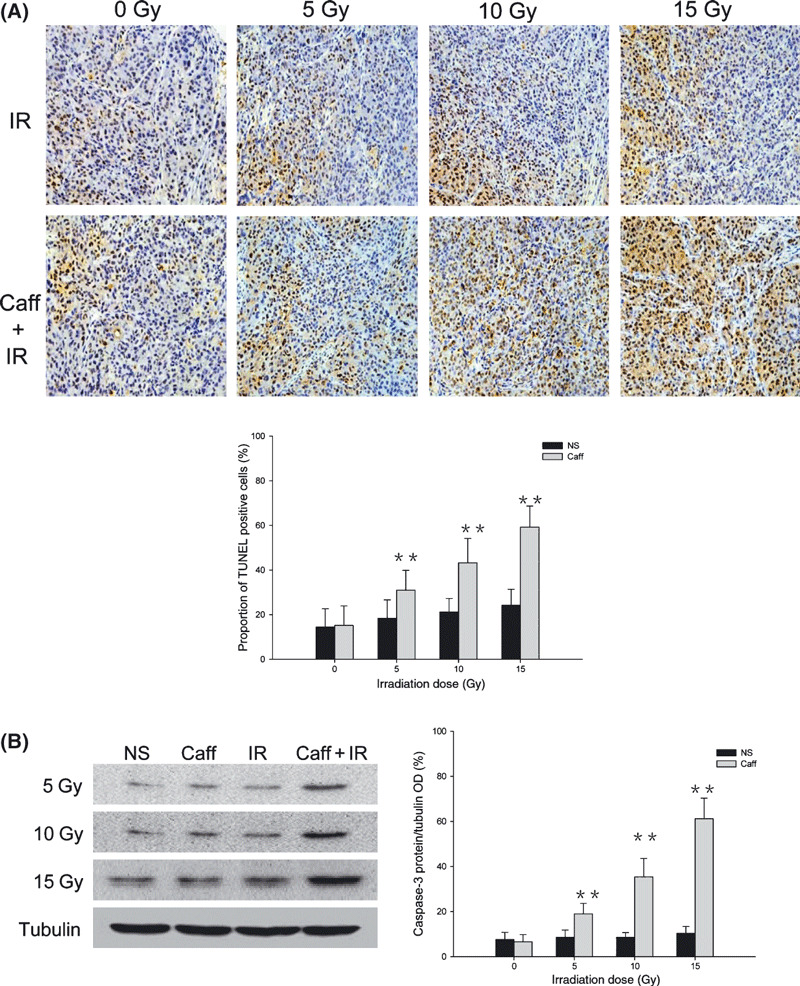
Caffeine (Caff) enhanced apoptosis to ionizing irradiation (IR) in LM3 hepatocellular carcinoma cells. (A) Results of TUNEL assay at 0 Gy, 12 h after 5‐Gy, 10‐Gy, and 15‐Gy IR with or without caffeine. The number of positive cells in 10 randomly selected areas was counted under the microscope (original magnification, ×200). (B) Western blot analysis of caspase‐3 expression at 0 Gy, 12 h after 5‐Gy, 10‐Gy, and 15‐Gy IR with or without caffeine. Data are expressed as the mean ± SD (n = 5 per group). NS, normal saline. *P < 0.05 compared with the IR alone group; **P < 0.01 compared with the IR alone group.
We used immunoblotting to assess apoptosis by measuring caspase‐3 expression level as a marker (Fig. 6B). Consistent with the TUNEL staining results, caspase‐3 protein expression in the NS group was not significantly different from that of the Caff group, and caspase‐3 protein expression was significantly higher in the Caff + IR group compared with the IR alone group at the different IR doses. Caspase‐3 expression increased with increasing IR dose and caffeine dose in the Caff + IR group, whereas caspase‐3 expression did not change significantly in the IR alone group with different IR doses.
Discussion
In this study, we found that caffeine improved the antitumor effect of IR in LM3 human hepatocellular carcinoma in vitro and in vivo, in which survival of cells was decreased and tumor growth was inhibited. The IC50 of caffeine was 2.89 mM for the LM3 cell line, as measured by the MTT assay at a previous preliminary experiment, so the concentration of 2.5 mM was used for optimal radiosensitization for our study. In the in vitro study, G2 phase arrest reached a peak at 12 h after IR, and caffeine reversed this effectively. As a result, we chose the time point of 12 h after IR to study the mechanism of caffeine radiosensitization in the in vivo experiments. Radiation increased CDC2‐Tyr15‐P protein expression, and caffeine inhibited this increase. At the same time, IR significantly reduced cyclinB1 protein expression, and caffeine restored it. Interestingly, the effect of caffeine on reducing expression of CDC2‐Tyr15‐P and restoring expression of cyclinB1 was independent of IR dose, which should be helpful in maintaining abrogation of G2 phase arrest during radiotherapy. The fact that CDC2‐Tyr15‐P and cyclinB1 protein expression levels were similar in the IR alone group and the Caff + IR group at 36 h after the first IR suggested that if caffeine was given again after 5‐Gy IR, it could effectively maintain the low expression of CDC2‐Tyr15‐P protein and the high expression of cyclinB1 protein. The caffeine concentration and frequency of administration in the setting of hypofractionated IR improved the radiosensitivity of LM3 human hepatocellular cancer, which may provide some reference for future clinical applications.
In addition, we found that abrogation of G2 phase arrest that leads to increased radiosensitivity is linked with enhancement of IR‐induced apoptosis, as indicated by TUNEL‐positive cells and caspase‐3 activity. Interestingly, the proportion of TUNEL‐positive cells did not change with increasing IR dose in the IR group. It may be that although IR‐induced G2 phase arrest gradually increased, as indicated by the gradual decrease in CDC2/cyclinB1 complex activity with increasing IR dose, this provided enough time for DNA repair. In contrast, in the Caff + IR group, the proportion of TUNEL‐positive cells increased significantly with increasing IR dose and caffeine dose. This may be explained by the fact that caffeine maintained similar levels of low expression of CDC2‐Tyr15‐P and high expression of cyclinB1 after different IR doses, thus effectively maintaining abrogation of G2 phase arrest during IR. High caspase‐3 expression is thought to be an important factor for inducing apoptosis, and disturbance of caspase‐3 activation has been implicated in failure of anticancer therapies.( 25 , 26 ) We were pleased to find that caffeine increased caspase‐3 expression of LM3 human hepatocellular cancer after IR, further affirming that caffeine enhanced the anticancer effect of IR. Based on our results, we conclude that caffeine could abolish IR‐induced G2 phase arrest, eventually leading to apoptosis of LM3 hepatocellular carcinoma in vivo. Enhancement of radiosensitivity is perhaps due to the decrease in DNA repair time during the G2 period. This hypothesis is consistent with the results of many in vitro studies.( 4 , 5 )
DNA lesions trigger cell‐cycle arrest, which provides time to repair the IR‐induced DNA damage and maintain genomic integrity.( 7 , 27 , 28 ) In general, cells expressing wild‐type p53 arrest in the G1 phase, whereas cells that do not express p53 or express mutant p53 arrest mainly in the G2 phase.( 29 ) As mutations in the p53 gene are present in more than half of all human tumors,( 30 ) the majority of those tumor cells will undergo G2 phase arrest as a result of ionizing IR, and this might be an important mechanism of resistance to radiotherapy that develops in many cancers. The effect of caffeine seems to be most potent in p53‐deficient cells,( 4 , 31 ) but it had no effect on G2 phase delay in normal cell lines with wild‐type p53.( 8 ) This seems to underscore the potential of caffeine as a tumor radiosensitizing agent, because ideally, the radiosensitizing agent would not aggravate damage of normal tissue around the tumor.
Although the radiosensitization effect of caffeine might determine its clinical application in radiotherapy, caffeine has not been used in clinical practice as a radiosensitizing agent because patients cannot tolerate the millimolar concentrations required for an effective dose of caffeine in vitro. However, caffeine is almost 100% bioavailable when taken orally,( 21 ) it is absorbed by the stomach and small intestine promptly after ingestion, and it accumulates rapidly in the portal vein to reach the liver and become concentrated in liver tumor tissue. Furthermore, caffeine is metabolized in the liver. Consequently, it is possible that a low dose could significantly increase the radiosensitivity of hepatocellular cancer compared with other types of cancer. This was also an important reason why we chose gastrointestinal administration of caffeine in this study. Normal liver tissue is susceptible to damage from IR, with the threshold dose for whole liver IR of be 20–30 Gy.( 32 , 33 ) Consequently, IR‐induced liver injury limits the radiotherapy dose in hepatocellular cancer, which might contribute to hepatocellular cancer recurrence after radiotherapy. Adding caffeine may further boost IR treatment efficacy and permit reduction of the IR dose, especially for tumors of large size.
The addition of caffeine to radiotherapy for hepatocellular cancer has tremendous clinical potential. Nevertheless, a number of questions are still to be resolved.
-
1
Will normal liver tissue be affected by caffeine in vivo?
-
2
What dose of caffeine will obtain the best treatment benefit? In the present study, caffeine (100 mg/kg body weight) showed effective radiosensitization; would it be effective if the caffeine dose was reduced?
-
3
Can we attenuate the chemical toxicity of caffeine, for example, by enclosure in liposomes?
-
4
The hypofractionated treatment we chose in combination with caffeine was effective, but what about the fractionated IR regimens conventionally used in clinical practice? Which is better? Thus, further study is needed.
In conclusion, due to its pharmacokinetic characteristics, caffeine was effective as a radiosensitizing agent for human hepatocellular cancer in a nude mouse model. These results support the possibility of further clinical application.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Mornex F, Girard N, Beziat C et al. Feasibility and efficacy of high‐dose three‐dimensional‐conformal radiotherapy in cirrhotic patients with small‐size hepatocellular carcinoma non‐eligible for curative therapies – mature results of the French Phase II RTF‐1 trial. Int J Radiat Oncol Biol Phys 2006; 66: 1152–8. [DOI] [PubMed] [Google Scholar]
- 3. Zeng ZC, Tang ZY, Fan J et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J (Sudbury, Mass.) 2004; 10: 307–16. [DOI] [PubMed] [Google Scholar]
- 4. Higuchi K, Mitsuhashi N, Saitoh J et al. Caffeine enhanced radiosensitivity of rat tumor cells with a mutant‐type p53 by inducing apoptosis in a p53‐independent manner. Cancer Lett 2000; 152: 157–62. [DOI] [PubMed] [Google Scholar]
- 5. Yao SL, Akhtar AJ, McKenna KA et al. Selective radiosensitization of p53‐deficient cells by caffeine‐mediated activation of p34cdc2 kinase. Nat Med 1996; 2: 1140–3. [DOI] [PubMed] [Google Scholar]
- 6. Palayoor ST, Macklis RM, Bump EA, Coleman CN. Modulation of radiation‐induced apoptosis and G2/M block in murine T‐lymphoma cells. Radiat Res 1995; 141: 235–43. [PubMed] [Google Scholar]
- 7. Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003; 22: 5834–47. [DOI] [PubMed] [Google Scholar]
- 8. Jha MN, Bamburg JR, Bernstein BW, Bedford JS. Caffeine eliminates gamma‐ray‐induced G2‐phase delay in human tumor cells but not in normal cells. Radiat Res 2002; 157: 26–31. [DOI] [PubMed] [Google Scholar]
- 9. Sarkaria JN, Busby EC, Tibbetts RS et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 1999; 59: 4375–82. [PubMed] [Google Scholar]
- 10. Taylor YC, Parsian AJ, Duncan PG. Differential post‐irradiation caffeine response in normal diploid versus SV40‐transformed human fibroblasts: potential role of nuclear organization and protein‐composition. Int J Radiat Biol 1993; 64: 57–70. [DOI] [PubMed] [Google Scholar]
- 11. Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of CDC2 following DNA replication block and radiation‐induced damage in human cells. Mol Biol Cell 1997; 8: 1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation‐induced G2 arrest in human cells. J Cell Biol 1996; 134: 963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta 2001; 1519: 1–12. [DOI] [PubMed] [Google Scholar]
- 14. Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol 1994; 6: 877–82. [DOI] [PubMed] [Google Scholar]
- 15. Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev 1997; 11: 504–11. [DOI] [PubMed] [Google Scholar]
- 16. Qi W, Qiao D, Martinez JD. Caffeine induces TP53‐independent G(1)‐phase arrest and apoptosis in human lung tumor cells in a dose‐dependent manner. Radiat Res 2002; 157: 166–74. [DOI] [PubMed] [Google Scholar]
- 17. Theron T, Bohm L. Cyclin B1 expression in response to abrogation of the radiation‐induced G2/M block in HeLa cells. Cell Prolif 1998; 31: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muschel RJ, Zhang HB, Iliakis G, McKenna WG. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res 1991; 51: 5113–7. [PubMed] [Google Scholar]
- 19. Bernhard EJ, Maity A, Muschel RJ, McKenna WG. Increased expression of cyclin B1 mRNA coincides with diminished G2‐phase arrest in irradiated HeLa cells treated with staurosporine or caffeine. Radiat Res 1994; 140: 393–400. [PubMed] [Google Scholar]
- 20. Murnane JP. Cell cycle regulation in response to DNA damage in mammalian cells: a historical perspective. Cancer Metastasis Rev 1995; 14: 17–29. [DOI] [PubMed] [Google Scholar]
- 21. Blanchard J, Sawers SJ. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol 1983; 24: 93–8. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Tang Y, Ye L et al. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis‐related genes through cDNA microarray. J Cancer Res Clin Oncol 2003; 129: 43–51. [DOI] [PubMed] [Google Scholar]
- 23. Yang BW, Liang Y, Xia JL et al. Biological characteristics of fluorescent protein‐expressing human hepatocellular carcinoma xenograft model in nude mice. Eur J Gastroenterol Hepatol 2008; 20: 1077–84. [DOI] [PubMed] [Google Scholar]
- 24. Sun FX, Tang ZY, Lui KD et al. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer 1996; 66: 239–43. [DOI] [PubMed] [Google Scholar]
- 25. Friedrich K, Wieder T, Von Haefen C et al. Overexpression of caspase‐3 restores sensitivity for drug‐induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene 2001; 20: 2749–60. [DOI] [PubMed] [Google Scholar]
- 26. Prokop A, Wieder T, Sturm I et al. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl‐2 ratio and loss of spontaneous caspase‐3 processing in vivo. Leukemia 2000; 14: 1606–13. [DOI] [PubMed] [Google Scholar]
- 27. Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature 2000; 408: 433–9. [DOI] [PubMed] [Google Scholar]
- 28. Paulovich AG, Toczyski DP, Hartwell LH. When checkpoints fail. Cell 1997; 88: 315–21. [DOI] [PubMed] [Google Scholar]
- 29. Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett 2007; 247: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopez‐Saez JF, De La Torre C, Pincheira J, Gimenez‐Martin G. Cell proliferation and cancer. Histol Histopathol 1998; 13: 1197–214. [DOI] [PubMed] [Google Scholar]
- 31. Powell SN, DeFrank JS, Connell P et al. Differential sensitivity of p53(−) and p53(+) cells to caffeine‐induced radiosensitization and override of G2 delay. Cancer Res 1995; 55: 1643–8. [PubMed] [Google Scholar]
- 32. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995; 31: 1237–48. [DOI] [PubMed] [Google Scholar]
- 33. Antoch G, Kaiser GM, Mueller AB et al. Intraoperative radiation therapy in liver tissue in a pig model: monitoring with dual‐modality PET/CT. Radiology 2004; 230: 753–60. [DOI] [PubMed] [Google Scholar]


