Figure 4.
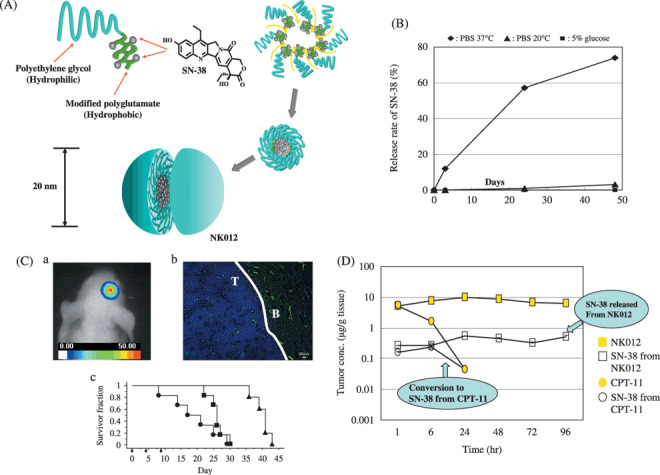
(A) Schematic structure of NK012. A polymeric micelle carrier of NK012 consists of a block copolymer of polyethylene glycol (PEG) (molecular weight of ≈ 5000) and partially modified polyglutamate (≈
5000) and partially modified polyglutamate (≈ 20 units). Polyethylene glycol (hydrophilic) is believed to be the outer shell and SN‐38 was incorporated into the inner core of the micelle. (B) The releasing rates of 7‐ethyl‐10‐hydroxy‐CPT (SN‐38) from NK012. The data suggested that NK012 is stable in 5% glucose solution before administration and starts to release SN‐38, gradually, under physiological conditions after administration. (C) (a) An orthotopic glioma model. Twenty days after U87MG/Luc inoculation. (b) The orthotopic tumor was visualized using a photon imager. The maximum tolerated dose (MTD) of NK012 (30 mg/kg) or Irinotecan hydrochloride (CPT‐11) (66.7 mg/kg) was injected intravenously into the tail vein of mice. 24 h after NK012 injection, mice were also administered with fluorescein Lycopersicon esculentum lectin (100 µL/mouse) to visualize tumor blood vessels (green). NK012 (blue) was accumulated selectively in tumor tissue. T: tumor, B: normal brain (c) NK012 (30 mg/kg/day), CPT‐11 (66.7 mg/kg/day) (
20 units). Polyethylene glycol (hydrophilic) is believed to be the outer shell and SN‐38 was incorporated into the inner core of the micelle. (B) The releasing rates of 7‐ethyl‐10‐hydroxy‐CPT (SN‐38) from NK012. The data suggested that NK012 is stable in 5% glucose solution before administration and starts to release SN‐38, gradually, under physiological conditions after administration. (C) (a) An orthotopic glioma model. Twenty days after U87MG/Luc inoculation. (b) The orthotopic tumor was visualized using a photon imager. The maximum tolerated dose (MTD) of NK012 (30 mg/kg) or Irinotecan hydrochloride (CPT‐11) (66.7 mg/kg) was injected intravenously into the tail vein of mice. 24 h after NK012 injection, mice were also administered with fluorescein Lycopersicon esculentum lectin (100 µL/mouse) to visualize tumor blood vessels (green). NK012 (blue) was accumulated selectively in tumor tissue. T: tumor, B: normal brain (c) NK012 (30 mg/kg/day), CPT‐11 (66.7 mg/kg/day) ( ) and saline (
) and saline ( ) were intravenously given on days 0 (20 days after tumor inoculation), 4, and 8 (
) were intravenously given on days 0 (20 days after tumor inoculation), 4, and 8 ( ). Kaplan‐Meier analysis was performed to determine the effect of drugs on time to morbidity, and statistical differences were ranked according to the Mantel‐Cox log‐rank test using StatView 5.0. (D) Intra‐tumor distribution of CPT‐11, NK012 (or polymer bound SN‐38), and free SN‐38 after administration of NK012 and CPT‐11 to mice bearing Capan1 xenografts. The time profiles of polymer‐bound SN‐38 (
). Kaplan‐Meier analysis was performed to determine the effect of drugs on time to morbidity, and statistical differences were ranked according to the Mantel‐Cox log‐rank test using StatView 5.0. (D) Intra‐tumor distribution of CPT‐11, NK012 (or polymer bound SN‐38), and free SN‐38 after administration of NK012 and CPT‐11 to mice bearing Capan1 xenografts. The time profiles of polymer‐bound SN‐38 ( ), free SN‐38 released from NK012 (
), free SN‐38 released from NK012 ( ), CPT‐11 (
), CPT‐11 ( ), and free SN‐38 converted from CPT‐11 (
), and free SN‐38 converted from CPT‐11 ( ) were obtained by high performance liquid chromatography (HPLC) analysis. The time‐points examined were 1, 6, 24, 48, 72, and 96 h after the administration of CPT‐11 or NK012.
) were obtained by high performance liquid chromatography (HPLC) analysis. The time‐points examined were 1, 6, 24, 48, 72, and 96 h after the administration of CPT‐11 or NK012.
