Figure 2.
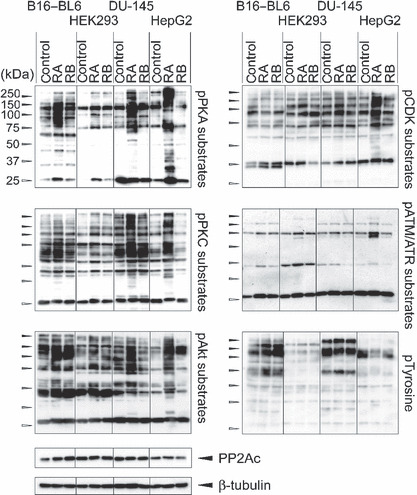
Overphosphorylation of proteins in rubratoxin A‐treated cells. Logarithmically‐growing B16–BL6, HEK293, DU‐145, and HepG2 cells were treated with 20 μm rubratoxins (rubratoxin A [RA] or rubratoxin B [RB]) or vehicle (control; 0.07% MeCN) for 3 h. Cell lysates containing 10 μg protein were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis, and the phosphorylation states of proteins were analyzed by immunoblotting using antibodies recognizing the phosphorylated substrates of protein kinase A (pPKA), protein kinase (pPKC), Akt, cyclin‐dependent kinase (pCDK), ataxia telangiectasia mutated ataxia telangiectasia mutated and rad3‐related protein (pATM/pATR), and phosphorylated tyrosine (pTyrosine). Expression level of the protein phosphatase (PP)2A catalytic subunit and β‐tubulin were detected as the controls.
