Figure 1.
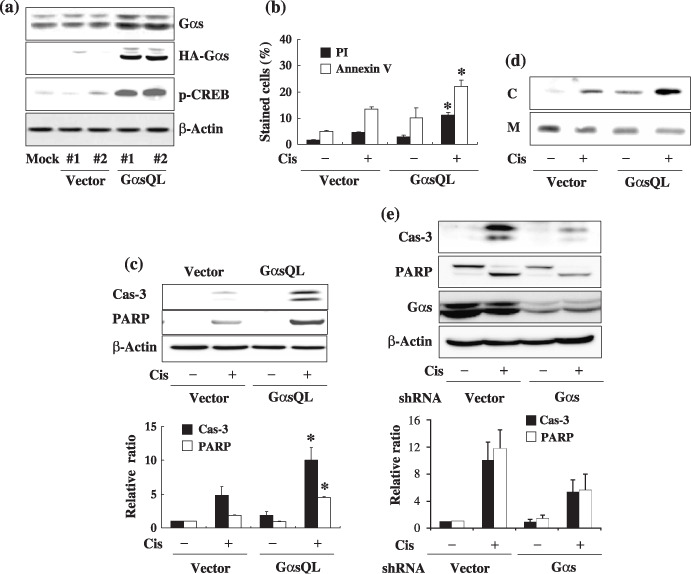
Alpha subunit of stimulatory heterotrimeric GTP‐binding protein (Gαs) augments cisplatin‐induced apoptosis of A549 human lung cancer cells. (a) Stable expression of alpha subunit of stimulatory heterotrimeric GTP‐binding protein Q227L (GαsQL) in A549 cells. Expression of GαsQL and phosphorylation of cyclic AMP response element binding protein (CREB) in hemaglutinin (HA)‐tagged GαsQL‐transfected cells were confirmed by western blot analysis using a specific antibody against Gαs, HA, or phosphorylated CREB (Ser‐133). β‐Actin was analyzed as a loading control; #1 and #2 are the respective clone numbers of vector‐ or GαsQL‐transfected cells. (b–d) Assessment of cisplatin‐induced apoptosis of A549 cells by (b) flow cytometry of annexin V‐ and propidium iodide (PI)‐stained cells, (c) western blot analysis of caspase‐3 and poly (ADP‐ribose) polymerase (PARP) cleavage, and (d) cytosolic release of cytochrome c (C, cytosolic fraction; M, mitochondrial fraction). A549 cells were then treated with 40 µM cisplatin for 24 h prior to assessment of apoptosis. (e) Knockdown of Gαs expression decreased cisplatin‐induced apoptosis. Subconfluent A549 cells were transfected with Gαs small hairpin RNA (shRNA), and 48 h after transfection cells were treated with 40 µM cisplatin for 24 h, after which apoptosis was assessed by western blot analysis of caspase‐3 and PARP cleavage. Histograms represent mean ± SE, and the asterisks indicate a significant difference from vector‐transfected controls (P < 0.05, Mann–Whitney U‐test).
