Figure 2.
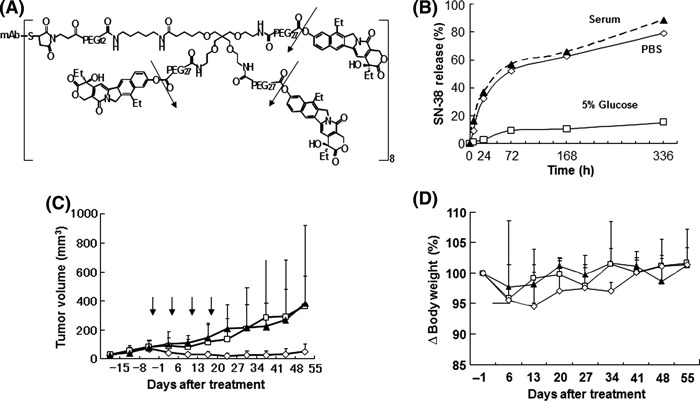
Drug design, anti‐tumor effect and pharmacokinetics of the anti‐fibrin immunoconjugate. (A) Drug design of the immunoconjugate: mAb–PEG–three‐branched PEG–(SN‐38)3 linked via an ester bond. One antibody bears 24 molecules of SN‐38. The arrows indicate the cleavage sites to release free active SN‐38. (B) Release of SN‐38 from immunoconjugates over time in mouse serum, PBS (pH 7.4), or 5% glucose at 37°C. Data show single values determined at each time point. (C) Antitumor activity was examined in vivo. Immunoconjugates (⋄), camptothecin‐11 (CPT‐11; □), or saline (), were administered to mice bearing chemical‐induced cutaneous tumors via intravenous injection on Days 0, 7, 14, and 21. Arrows indicate the day of administration and the curves illustrate the effect of treatment on tumor size. Data are the mean ± SD (n = 5 in each group). P = 0.0005 for CPT‐11 compared with immunoconjugate; P < 0.0001 for saline compared with immunoconjugate. (D) Changes in the relative body weight of mice injected with Immunoconjugates (⋄), CPT‐11 (□), or saline () on Days 0, 7, 14, and 21. Data are the mean ± SD. There were no significant differences between the three groups (P = 0.09 for CPT‐11 versus immunoconjugate; P = 0.0866 for saline versus immunoconjugate).
