Abstract
Surgical treatment often causes difficulty in the irradiated field because of delayed wound healing, which is mainly due to vascular dysfunction. To overcome this difficulty, we attempted to accelerate the recovery from clamp injury in irradiated superficial epigastric arteries of rats as a model. Etanercept, a soluble receptor of tumor necrosis factor‐α, was administered four times to rats with irradiated arteries before and after clamp injury. Loss of endothelial cells and necrosis of the media in the irradiated arteries continued for more than 1 week after the injury; however, in the rats treated with etanercept, the endothelial cells recovered in the intima, and α‐smooth muscle actin‐positive smooth muscle cells recovered in the injured and irradiated arteries. After clamp injury of common carotid arteries that had previously been irradiated, the blood flow in these arteries was visualized by magnetic resonance (MR) angiography. The time‐of‐flight signal was weakened in the injured and irradiated arteries. This time‐of‐flight signal was recovered by the etanercept treatment. These findings suggest that etanercept improves the radiation‐impaired healing of arteries in rats. (Cancer Sci 2009)
Preoperative radiotherapy for cancer patients is often done for the purpose of downstaging cancers. Following cancer resectioning, constructive surgery is frequently carried out for patients with head and neck cancer, colon cancer, or soft tissue sarcoma.( 1 ) Anastomotic failure frequently occurs in these cases. Several factors, including wound complications, are considered to be causes.( 2 , 3 , 4 )
Small vessels, such as capillaries, arterioles, and venules, are targets for ionizing radiation but are rare in irradiated tissues.( 5 ) Furthermore, ischemia in the graft beds results from venous and arterial thrombosis,( 6 ) and large vessels are also damaged by ionizing radiation. Endothelial cell swelling and sloughing induced by radiation leads to luminal compromise and vessel thrombosis. Schultze‐Masgau et al. showed a decrease in the ratio of media area/total vessel area in arteries that had received preoperative radiation.( 7 ) Radiation arteritis occurs proximal to the atherosclerotic region of irradiated arteries.( 5 ) Beckman et al. suggested that impairment of endothelium‐dependent vasodilation results in arterial occlusion in irradiated arteries.( 8 ) Thus, inflammatory alterations of the vascular endothelium following preoperative radiotherapy may cause healing defects or delays in free flaps in irradiated graft beds. Perivascular fibrosis in the pedicles of these free flaps might also cause arterial thrombosis. Consequently, in a significant number of cancer patients, irradiated arteries are not available for flap transplantation. If the arteries of irradiated graft beds could be made more readily available to reconstructive surgery, more radical surgery might be safely carried out.
We have investigated radiation‐impaired wound healing in experimental animals. The impaired healing of cutaneous wounds in rats can be improved by a combination treatment involving granulocyte‐colony stimulating factor, macrophage‐colony stimulating factor, and a transforming growth factor β‐signal inhibitor, SB431542.( 9 ) In preliminary experiments using rats, more than 50 Gy of ionizing radiation induced arterial thrombosis after anastomosis of the superficial epigastric artery in rats. When 30–40 Gy of ionizing radiation was administered to rat arteries, the subsequent arterial anastomosis delayed recovery of endothelial cells and medial smooth muscle cells around the anastomotic regions of the irradiated arteries, which were associated with vasodilation (M. Takanashi, 2007, unpublished observation). These pathological features are similar to those in radiation arteritis, which is produced in regions proximal to atherosclerosis in humans.
Ionizing radiation activates NF‐κB signals associated with TNF‐α signal activation,( 10 , 11 ) and an increase in the level of TNF‐α is found in the wound fluid of irradiated rat skin.( 12 ) TNF‐α‐activated leukocytes promote endothelial cell death in the intima. Etanercept is a soluble TNF‐α receptor fusion protein that is used to treat rheumatoid arthritis in humans.( 13 ) Etanercept treatment protects against lipopolysaccharide‐induced rolling and adhesion of leukocytes to the retinal artery, and vascular leakage in an endotoxin‐induced uveitis model in rats.( 14 ) Thus, TNF‐α signals may be a target for healing therapy in irradiated fields. We selected etanercept to accelerate the wound healing after surgical injury in the irradiated field.
In the preliminary experiments, experimental clamp injuries produced pathological features similar to those seen in the anastomosis of irradiated arteries in rats. Thus, we tried to establish a therapy to improve the radiation‐induced impairment of the healing of injuries in rat arteries by etanercept treatment.
Materials and Methods
Animals. Female 4‐week‐old F344 rats were purchased from Japan SLC Inc. (Hamamatsu Shizuoka, Japan) and given ad libitum access to food and water. The animal experiments were carried out in accordance with the Guidelines of the Animal Care and Use Committee of the National Cancer Center.
Radiation‐induced healing delay model in superficial epigastric artery. The right groin of rats (n = 12) was exposed to X‐ray radiation (7.5 Gy, 150 kV) in a Faxitron Cabinet X‐ray System, Model CP‐160 (Faxitron X‐ray Corporation, Lincolnshire, IL, USA) five times for 4 weeks. Another part of the body was covered with a lead plate (5 mm thick) in each anesthetized rat. Non‐irradiated rats (n = 4) were also prepared for surgical wounding.
Four weeks after the final irradiation, the superficial epigastric arteries of both groins were injured by placing four vascular clamps (total 8 mm length) side by side for 10 min, according to the method of Jackiewicz et al.( 15 ) One week after the operation, the healing of the artery was histologically investigated.
X‐ray irradiation and creation of a surgical wound in the common carotid artery. To evaluate arterial blood flow using MR angiography, the neck of the rats (eight rats) was irradiated with X‐rays (37.5 Gy, five fractions for 4 weeks) using the same procedure as in the superficial epigastric artery. Four weeks later, the right common carotid artery was injured with four clamps (~8 mm width) for 1 h. Because the common carotid artery is elastic type (the superficial epigastric artery is of the muscular type), the clamp injury was carried out for a long period. Non‐irradiated rats (n = 3) were injured using the same procedures as irradiated rats. Four weeks after the operation, the rats underwent MR imaging examinations, followed by histological analysis.
Administration of etanercept to heal arterial injuries. Etanercept (ENBREL) was purchased from Wyeth/Takeda Chemical Industries, Shinagawa, Tokyo, Japan, and dissolved in sterile water. In the healing model of the superficial epigastric artery, etanercept (0.3 or 1.0 µg/kg) was subcutaneously injected into the abdominal skins of irradiated rats (each n = 4) on preoperative days 3 and 1, and postoperative days 1 and 3. The same volume of saline was injected into other irradiated rats as controls (n = 4). Non‐irradiated rats (n = 4) were also injected with saline.
In the healing model of the common carotid artery, etanercept (0.3 µg/kg) was subcutaneously injected into the abdominal skin of four irradiated rats on preoperative days 3 and 1, and postoperative days 1, 3, 7, 14, and 21. The same volume of saline was injected into another four irradiated rats as controls. Non‐irradiated rats (n = 3) were also injected with saline.
MR angiography of common carotid arteries. All MR images were taken using a 3.0Tesla whole body scanner (Signa HDx 3.0T; GE Medical System, Milwaukee, WI, USA) equipped with an in‐house‐built solenoid coil (35 mm in diameter). A three‐dimensional TOF MRA sequence (repetition time/echo time/flip angle = 24/5.8 ms/20°) was used to obtain carotid angiograms with a 34 mm field of view, a slice thickness of 0.8 mm, a 512 × 256 matrix, and two excitations. The number of stenotic regions in the right (injured) common carotid artery, which were shown as areas of low signal intensity, was counted on the MIP images that were reconstructed from the MRA data set.
Histological examination. The rats were perfused with 2% paraformaldehyde in PBS, and then the superficial epigastric or common carotid arteries were removed and fixed with 2% paraformaldehyde in PBS for another 3 h. The arteries were washed with PBS, submerged into 20% sucrose‐containing PBS, and then embedded with OCT compound (Sakura Fine Technical Co., Tokyo, Japan). Frozen sections (8–10 µm thickness) of the arteries were stained with HE or Elastica van Gieson and used for immunological staining.
Immunological staining. Endothelial cells were immunostained with mouse monoclonal antibody to RECA‐1 (1:200; Abcam, Inc., Cambridge, MA, USA). Smooth muscle cells in the media were immunostained with mouse monoclonal antibody to α‐SMA (1:600; Sigma Chemical Co., St Louis, MO, USA). Laminin and collagen IV in the basement membrane of the artery were immunostained with rabbit polyclonal antibodies to laminin (1:100; Sigma Chemical Co.) and collagen IV (1:1000; Immune Systems Ltd., Paignton, Devon, UK) respectively.
For double staining in frozen sections, the sections were exposed to the primary antibodies for 1 h, followed by exposure to Alexa Fluor 488‐labeled anti‐rabbit IgG (1:400; Molecular Probes, Inc., Eugene, OR, USA), Alexa Fluor 633‐labeled anti‐mouse IgG (1:400; Molecular Probes), and PI (10 µg/mL; Sigma) for 30 min. Fluorescence was detected using a confocal laser scanning microscope (LSM 5 PASCAL; Carl Zeiss, Inc., Jena, Germany). The number of endothelial cells was determined from the number of RECA‐1‐positive cells in the intima. The amounts of laminin in the basement membrane of the artery and α‐SMA in the media were estimated from the sum of the signal intensities (levels 200–255) in the corresponding regions of the arteries.
Statistical analysis. The results of the quantitative analyses were expressed as means ± SEM. Differences were considered to be statistically significant at a P‐value of 0.05 according to Student's t‐test. The statistical analyses were carried out with StatView version 5 software (Abacus‐concepts; Piscataway, NJ, USA) on a Macintosh computer.
Results
Overexpression of laminin in the basement membrane of the superficial epigastric arteries after irradiation followed by clamp injury. First, laminin was immunohistologically stained in frozen sections of the irradiated superficial epigastric arteries 1 week after clamp injury. Overexpression of laminin in the basement membrane of the intima was found in the irradiated arteries after the clamp injury (Fig. 1B), compared with that of the non‐injured or ‐irradiated arteries (Fig. 1A). This was associated with loss of endothelial cells in the intima (Fig. 1B). Collagen IV was also largely overexpressed in the basement membrane of the injured or irradiated arteries (Fig. 1D), compared with that of the non‐injured or ‐irradiated arteries (Fig. 1C). The overexpression of laminin was caused by clamp injury in irradiated arteries (Fig. 1E).
Figure 1.
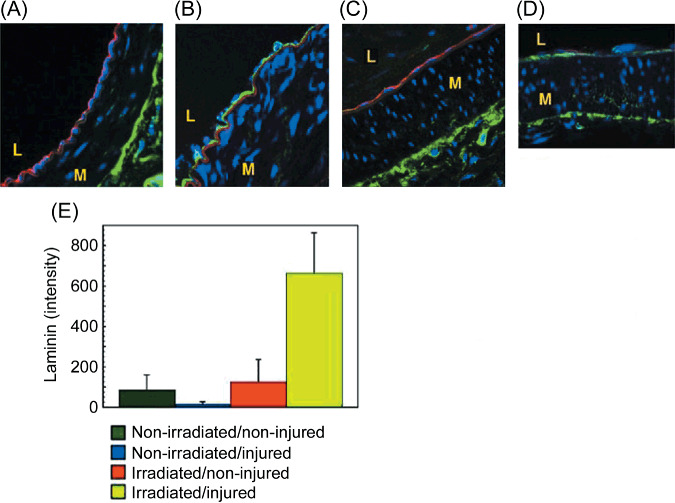
The accumulation of laminin in the basement membrane of injured or irradiated superficial epigastric arteries. The right groin of the rats was irradiated with fractionated X‐ray irradiation (total 37.5 Gy). Four weeks later, the superficial epigastric arteries of both groins were injured with four vascular clamps (8 mm length) for 10 min. One week after the injury, each rat was perfused with 2% paraformaldehyde in PBS. Then, the arteries were removed and fixed for 3 h. Frozen sections of the arteries were stained with antibody against rat endothelial cell antigen (RECA)‐1, laminin, or collagen IV. Nuclei were stained with propidium iodide (blue). (A) RECA‐1‐positive endothelial cells (red) and laminin (green) in an injured/non‐irradiated artery. (B) RECA‐1‐positive endothelial cells (red) and laminin (green) in an injured/irradiated artery. Note: the internal elastic lamina is non‐specifically stained (red). No RECA‐1‐positive endothelial cell was found in the intima. (C) RECA‐1‐positive endothelial cells (red) and collagen IV (green) in an injured/non‐irradiated artery. (D) RECA‐1‐positive endothelial cells (red) and collagen IV (green) in an injured and irradiated artery. Note: no RECA‐1‐positive endothelial cell was found in the intima. (E) The laminin content of the basement membrane of the superficial epigastric arteries. L, lumen; M, media. The original magnifications are ×630 in all images.
As these results suggest enhancement of the TNF‐α signal in the injured or irradiated artery, etanercept, an inhibitor of the TNF‐α signal, was used as a therapeutic agent to remodel the irradiated artery after injury.
Effect of etanercept on recovery from clamp injury in irradiated superficial epigastric artery. Etanercept was injected subcutaneously before and after the operative injury in irradiated rats. Elastica van Gieson staining showed loss of endothelial cells and necrosis of the media in the injured or irradiated arteries of the saline‐treated rats 1 week after the injury (Fig. 2B). In addition, internal elastic laminas were broken, and vasodilation was detected in the injured or irradiated arteries of the saline‐treated rats (Fig. 2B). These abnormalities indicated that arteritis had been induced by the clamp injuries in the irradiated arteries. In the irradiated rats treated with etanercept (0.3 or 1.0 mg/kg), the morphology of the injured or irradiated arteries was recovered and was similar to that in the non‐injured or ‐irradiated arteries (Fig. 2A,C).
Figure 2.
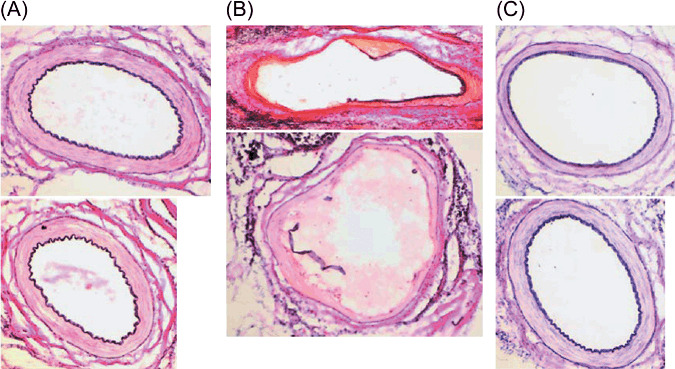
The effect of etanercept treatment on the morphology of injured/irradiated superficial epigastric arteries. The right superficial epigastric artery was irradiated and injured as described in Figure 1. The left superficial epigastric artery was not irradiated, but was injured. Etanercept (0.3 or 1.0 mg/kg bodyweight) was injected subcutaneously on day 3 as well as 1 day before, and days 1 and 3 after the clamp injury (each n = 4). As controls, the same volume of saline was injected subcutaneously into the injured/irradiated rats (n = 4) at the same time as the etanercept treatment. Frozen sections of the arteries were stained with Elastica van Gieson. (A) Typical images of injured/non‐irradiated arteries stained with Elastica van Gieson. (B) Typical images of injured/irradiated arteries stained with Elastica van Gieson in saline‐treated rats. (C) Typical images of injured/irradiated arteries stained with Elastica van Gieson in rats treated with etanercept (0.3 mg/kg). All original magnifications are ×100.
The number of endothelial cells in the injured or irradiated arteries decreased to one‐fifth of that of the non‐irradiated arteries in the saline‐treated rats (Fig. 3A,D). However, in the rats treated with etanercept 0.3 and 1.0 mg/kg, the number of endothelial cells in the injured or irradiated arteries recovered to a normal level (P < 0.03; Fig. 3B–D).
Figure 3.
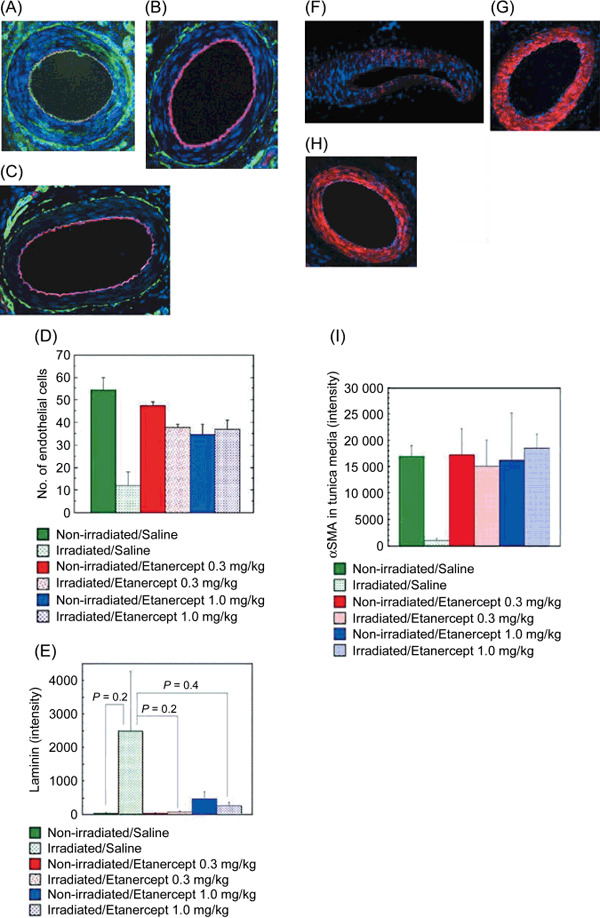
Enhanced healing of clamp injuries by etanercept treatment in irradiated superficial epigastric arteries. The right superficial epigastric artery was irradiated and injured as described in Figure 1. Etanercept treatment was carried out as described in Figure 2. Frozen sections of the arteries were immunostained with antibodies against rat endothelial cell antigen (RECA)‐1, laminin, and α‐smooth muscle actin (SMA). Nuclei were stained with propidium iodide (blue). The amount of α‐SMA in the media was estimated from the signal level obtained from confocal fluorescent microscopy. (A) RECA‐1‐positive endothelial cells (red) and laminin (green) in an injured/irradiated artery of a saline‐treated rat. Note: no RECA‐1‐positive endothelial cell was found in the intima. (B) RECA‐1‐positive endothelial cells (red) and laminin (green) in an injured/irradiated artery of a rat treated with etanercept (0.3 mg/kg). (C) RECA‐1‐positive endothelial cells (red) and laminin (green) in an injured/non‐irradiated artery of a rat treated with etanercept (0.3 mg/kg). (D) The number of endothelial cells counted from the number of RECA‐1‐positive cells in the intima. (E) The amount of laminin in the basement membrane, as estimated by confocal microscopy. (F) The α‐SMA (red) of an injured/irradiated superficial epigastric artery in a saline‐treated rat. (G) The α‐SMA (red) of an injured/irradiated superficial epigastric artery in a rat treated with etanercept (0.3 mg/kg). (H) The α‐SMA (red) of an injured/non‐irradiated superficial epigastric artery in a saline‐treated rat. (I) The amount of α‐SMA in the media, as estimated by confocal microscopy. All original magnifications are ×100.
The amount of laminin in the basement membrane of the arteries was measured by its intensity according to confocal microscopy. The increased amounts of laminin in the basement membranes of the injured or irradiated arteries in the saline‐treated rats were reduced to almost normal levels in the etanercept‐treated rats (Fig. 3E); however, the difference was not statistically significant (P = 0.2 or 0.4) because of the large standard error. Laminin also distributed in the media of the injured or irradiated arteries in the saline‐treated rats (Fig. 3A).
Recovery of the media after clamp injury was determined by α‐SMA staining. The amount of α‐SMA in the media of the irradiated arteries decreased to approximately 6% of that in the non‐irradiated arteries of the saline‐treated rats (Fig. 3F,I). However, in the rats treated with etanercept, the amount of α‐SMA recovered to the same level as was observed in the non‐irradiated arteries (Fig. 3G–I). These effects of etanercept were confirmed by two independent experiments.
Abnormal blood flow during arterial recovery of injured or irradiated common carotid arteries in MR angiography. Next, functional analysis of the artery was carried out by MR angiography in injured or irradiated common carotid arteries. Changes in the blood flow in the common carotid artery were assessed by MIP images that were reconstructed from 3D TOF angiograms (Fig. 4A–C). The TOF signals in several regions of the right (injured) common carotid arteries were decreased compared with those in the left (non‐injured) common carotid arteries in the irradiated rats treated with saline (Fig. 4A). The TOF signal intensities in the right common carotid arteries were similar to those in the left common carotid arteries of the irradiated rats treated with etanercept (Fig. 4B) and the non‐irradiated rats (Fig. 4C). We counted the number of stenotic regions in each injured artery, which were shown as areas of low signal intensity on the MIP images (Fig. 4D). The number of these stenotic regions in the injured area (approximately 8 mm in length) was 2.5 and 0.6 in the irradiated rats treated with saline and etanercept respectively (P = 0.03). The stenotic regions showed 45–55% and 10–15% smaller pixel counts in the injured (right) common carotid arteries than in the non‐injured (left) common carotid arteries of the rats treated with saline and etanercept, respectively, in the axial sections of the TOF images (data not shown). Thus, the blood flow in the injured or irradiated common carotid arteries of the saline‐treated rats was abnormal, but the abnormality was recovered after treatment with etanercept.
Figure 4.
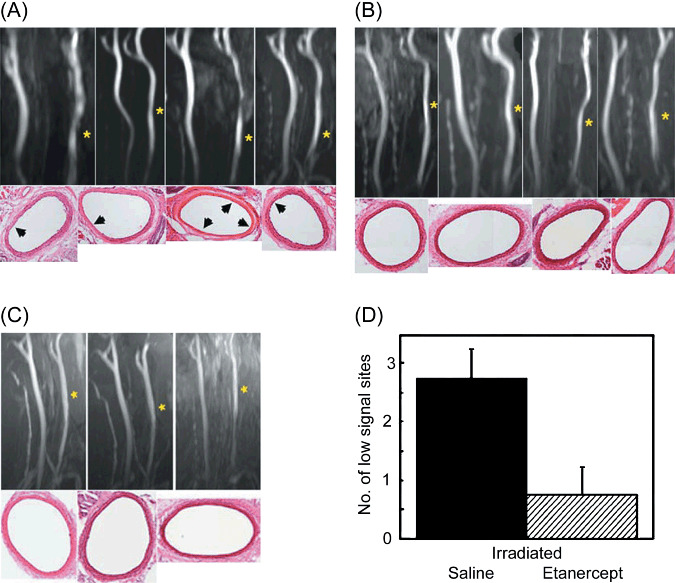
The effect of etanercept treatment on the functional recovery of injured/irradiated common carotid arteries. (A–C) The blood flow of the artery was visualized by 3D time‐of‐flight (TOF) angiograms. The neck of the rats was irradiated and the right common carotid artery was injured. The left common carotid artery was not injured and was used as a control. Etanercept (0.3 mg/kg bodyweight) was injected subcutaneously (n = 4). As controls, the same volume of saline was injected subcutaneously into the injured/irradiated rats (n = 4) at the same time as etanercept treatment. Three rats were not irradiated, but the right common carotid arteries of these rats were injured so that they could act as another control. The number of sites that showed low signal intensity in the injured artery was determined from TOF images of saline‐ or etanercept‐treated rats. (A) TOF figures and typical hematoxylin–eosin (HE)‐stained arteries of irradiated rats treated with saline. (B) TOF images and a typical HE‐stained artery of irradiated rats treated with etanercept. (C) TOF images and typical HE‐stained arteries of the non‐irradiated rats. The yellow asterisks in the TOF figures indicate the injured arteries. The black arrowheads indicate necrotic media in the HE‐stained arteries. All original magnifications in the HE‐stained arteries are ×100. (D) The number of sites showing low maximum intensity projection signal intensity in the injured arteries of the saline‐ or etanercept‐treated rats (each n = 4).
Decreased expression of α‐SMA in the media of the injured or irradiated common carotid arteries. The number of endothelial cells in the injured or irradiated common carotid arteries was not significantly different between the saline‐ and etanercept‐treated rats (P = 0.12, Fig. 5A). HE staining showed necrotic areas in the media of the injured or irradiated common carotid arteries of the saline‐treated rats (Fig. 4A). The amount of α‐SMA in the media of the injured or irradiated common carotid arteries in the etanercept‐treated rats was higher than that in the saline‐treated rats (P = 0.04, Fig. 5B). No stenosis was detected in a pathological analysis involving HE staining of the injured or irradiated arteries (Fig. 4A). Thus, the decreased blood flows may be related to depressed media function in the injured or irradiated common carotid arteries, and etanercept treatment can at least partially improve this functional aberration in irradiated common carotid arteries.
Figure 5.
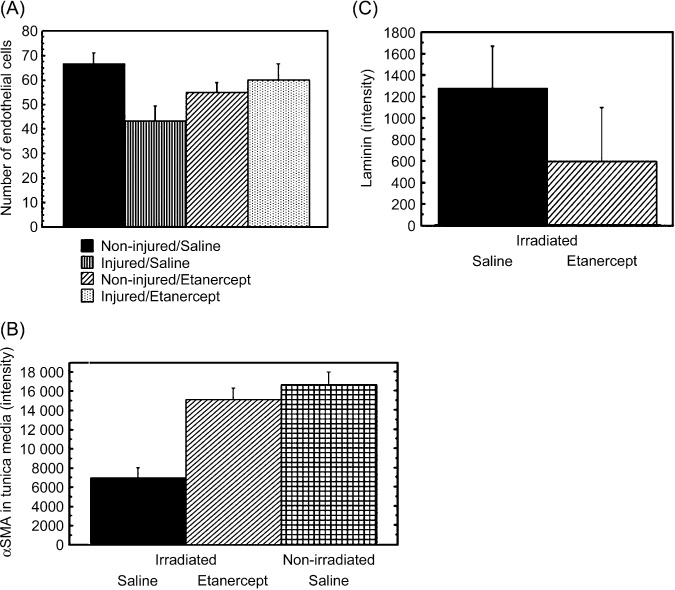
The effect of etanercept treatment on the healing of injured/irradiated common carotid arteries. Frozen sections of the arteries were stained with antibodies against rat endothelial cell antigen (RECA)‐1, α‐smooth muscle actin (SMA), and laminin. Nuclei were stained with propidium iodide (blue). The number of endothelial cells, and the amounts of α‐SMA in the media and laminin of the basement membrane were estimated from the number of RECA‐1‐positive cells and signal intensities obtained from confocal fluorescent microscopy respectively. (A) The number of endothelial cells in the injured/irradiated common carotid arteries. (B) The amount of α‐SMA in the media of the injured/irradiated common carotid arteries. (C) The amount of laminin in the basement membrane of the injured/irradiated common carotid arteries.
Discussion
In the present study, to prevent flap failure after reconstructive surgery in cancer patients that have undergone preoperative radiotherapy, we have tried to establish a therapeutic treatment to help improve the recovery from clamp injury‐induced arteritis in irradiated rat arteries. The experimental protocols were based on the conditions found in cases of cancer patients. We used an X‐ray dose of less than 40 Gy because arteries in fibrotic beds that have been previously irradiated with more than 50 Gy of ionizing radiation are generally not used for reconstructive surgery in cancer patients.( 2 , 3 , 16 ) Flap necrosis after preoperative radiotherapy is frequently associated with radiation arteritis in the patients.( 6 ) Clamp injury and arterial anastomosis both produce loss of endothelial cells and necrosis of the media, which showed arteritis in the rats. It was noted that the radiation doses used in this study did not induce arteritis without surgical injury. In clinical surgery, as the irradiated arteries in the radiation bed and the non‐irradiated arteries of flap pedicles are anastomosed, flap failure may be due to the irradiated arteries.
Because the overexpression of laminin in the basement membrane suggests enhancement of the TNF‐α signal in the injured or irradiated artery (it was reported that an enhanced TNF‐α signal is associated with loss of endothelial cells and abnormal accumulation of the intracellular matrix in arteriosclerosis( 17 ) and aging( 18 )), we chose etanercept, an inhibitor of the TNF‐α signal, as a therapeutic agent for remodeling of the injured or irradiated artery. The results indicated that etanercept treatment enhances healing of operative injuries in the irradiated arteries of rats. Etanercept improves periodontitis,( 19 ) endotoxin‐induced uveitis,( 14 ) and age‐related endothelial dysfunction, which are associated with TNF‐α signal activation( 18 ) and NF‐κB activation( 20 ) in experimental animals. Our experiments indicated that an etanercept dose of 0.3 mg/kg, four times with 2‐ or 3‐day intervals is sufficient to improve the recovery of radiation arteritis in rats (2, 3). This dose is similar to that suggested by previous reports.( 18 , 19 , 21 ) The side effects of etanercept were reported in cases of long‐term treatment of patients with rheumatoid arthritis;( 22 , 23 ) however, the etanercept in this study was administered for only a short period. Therefore, etanercept treatment for radiation arteritis may be beneficial for human clinical use.
Injuries in the intima and media of the irradiated superficial epigastric artery were both healed by etanercept treatment 1 week after clamp injury (2, 3). In the case of the injured or irradiated common carotid arteries 4 weeks after injury, the endothelial cells were almost completely recovered, whereas the media had hardly recovered at all, and etanercept improved healing of the media (Fig. 5). Therefore, etanercept may enhance the repair of smooth muscle cells in the media of injured or irradiated arteries as well as endothelial cells. During recovery of the intima, the accumulation of laminin in the basement membrane of the injured or irradiated arteries was not significantly decreased by etanercept treatment. Because the individual difference in the laminin content was large, further experiments using a higher number of rats are required.
In this study, although the endothelial cells in the irradiated common carotid arteries slowly recovered until 4 weeks after the clamp injury, no intimal hyperplasia was found (Fig. 4). In addition, recovery of the media was delayed (Fig. 4). A recent report indicated that radiation prevents intimal hyperplasia in human arteries that have been injured by surgery.( 24 ) Thus, the assumption in our experiments that radiation prevented regeneration of smooth muscle cells and intimal hyperplasia may be reasonable.
To clarify the functional abnormalities induced in injured or irradiated arteries, we carried out MR angiography (TOF) on the common carotid artery (Fig. 4A), as significant resolution of TOF images could not be obtained from the superficial epigastric arteries of the rats. The decrease in the TOF signal of the injured or irradiated arteries was similar to that seen in mild arterial stenosis in human cases. However, no stenosis was detected in a histological analysis of the irradiated arteries of the saline‐treated rats 4 weeks after the clamp injury. These decreases in TOF signals were associated with a decrease in the α‐SMA content of the media (Fig. 5). Thus, the abnormal signals of TOF images may have resulted from arterial flow abnormalities rather than structural stenosis, because the TOF MR angiography sensitively detects perturbations in the laminar flow of arterial blood. MR angiography is useful for detecting functional abnormalities in whole images of the small artery in small experimental animals. Because the amount of medial α‐SMA decreased in the injured or irradiated common carotid arteries in this period as mentioned above, the abnormal laminar flows of blood in these arteries may have resulted from healing defects in the media. The relationship between the abnormal laminar flow and medial necrosis should be clarified by further experiments.
In this study, although etanercept improved the healing of irradiated arteries, the effects of etanercept on the recovery of connective tissue and ischemia are not known. Attempts at functional recovery of whole tissues including free flaps will establish the usefulness of etanercept as a healing therapy. If etanercept treatment is effective after clinical surgery, the chances for radical surgery may increase for cancer patients with preoperative radiotherapy.
Abbreviations
| HE | hematoxylin–eosin |
| MIP | maximum intensity projection |
| MR | magnetic resonance |
| NF‐κB | nuclear factor‐κB |
| PI | propidium iodide |
| RECA | rat endothelial cell antigen |
| SMA | smooth muscle actin |
| TNF | tumor necrosis factor |
| TOF | time‐of‐flight |
Acknowledgments
The in‐house‐built solenoid coil (35 mm in diameter) used in MR angiography was provided for research by GE Yokogawa Medical Systems. This study was supported by Grants‐in‐Aid for Cancer Research from the Ministry of Health and Welfare and the Ministry of Education, Science, Sports, and Culture.
References
- 1. Ancona E, Ruol A, Castoro C et al . First‐line chemotherapy improves the resection rate and long‐term survival of locally advanced (T4, any N, M0) squamous cell carcinoma of the thoracic esophagus: final report on 163 consecutive patients with 5‐year follow‐up. Ann Surg 1997; 226: 714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eilber FR, Mirra JJ, Grant TT, Weisenburger T, Morton DL. Is amputation necessary for sarcomas? A seven‐year experience with limb salvage. Ann Surg 1980; 192: 431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindberg RD, Martin RG, Romsdahl MM, Barkley HT Jr. Conservative surgery and postoperative radiotherapy in 300 adults with soft‐tissue sarcomas. Cancer 1981; 47: 2391–7. [DOI] [PubMed] [Google Scholar]
- 4. Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5‐year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 2002; 94: 2256–64. [DOI] [PubMed] [Google Scholar]
- 5. Modrall JG, Sadjadi J. Early and late presentations of radiation arteritis. Semin Vasc Surg 2003; 16: 209–14. [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Qiu W, Mendenhall WM. Influence of radiation therapy on reconstructive flaps after radical resection of head and neck cancer. Int J Oral Maxillofac Surg 2003; 32: 35–8. [DOI] [PubMed] [Google Scholar]
- 7. Schultze‐Masgau S, Grabenbauer GG, Wehrhan F et al . Histomorphological structural changes of head and neck blood vessels after pre‐ or postoperative radiotherapy. Strahlenther Onkol 2002; 178: 299–306. [PubMed] [Google Scholar]
- 8. Beckman JA, Thakore A, Kalinowski BH, Harris JR, Creager MA. Radiation therapy impairs endothelium‐dependent vasodilation in humans. J Am Coll Cardiol 2001; 37: 761–5. [DOI] [PubMed] [Google Scholar]
- 9. Sugiyama K, Ishii G, Ochiai A, Esumi H. Improvement of the breaking strength of wound by combined treatment with recombinant human G‐CSF, recombinant human M‐CSF, and a TGF‐β1 receptor kinase inhibitor in rat skin. Cancer Sci 2008; 99: 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Natarajan M, Mohan S, Konopinski R, Otto RA, Herman TS. Induced telomerase activity in primary aortic endothelial cells by low‐LET gamma‐radiation is mediated through NF‐kappaB activation. Br J Radiol 2008; 81: 711–20. [DOI] [PubMed] [Google Scholar]
- 11. Hei TK, Zhou H, Ivanov VN et al . Mechanism of radiation‐induced bystander effects: a unifying model. J Pharm Pharmacol 2008; 60: 943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaffer M, Weimer W, Wider S et al . Differential expression of inflammatory mediators in radiation‐impaired wound healing. J Surg Res 2002; 107: 93–100. [PubMed] [Google Scholar]
- 13. Moreland LW, Baumgartner SW, Schiff MH et al . Treatment of rheumatoid arthritis with a recombinant tumor necrosis factor receptor (p75)–Fc fusion protein. N Engl J Med 1997; 337: 141–7. [DOI] [PubMed] [Google Scholar]
- 14. Koizumi K, Poulaki V, Doehmen S et al . Contribution of TNF‐α to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin‐induced uveitic in vivo . Invest Ophthalmol Vis Sci 2003; 44: 2184–91. [DOI] [PubMed] [Google Scholar]
- 15. Jackiewicz TA, McGeachie JK, Tennant M. Structural recovery of small arteries following clamp injury: a light and electron microscopic investigation in the rat. Microsurgery 1998; 17: 674–80. [DOI] [PubMed] [Google Scholar]
- 16. Eilber FR, Eckhardt J, Morton DL. Advances in the treatment of sarcomas of the extremity. Current status of limb salvage. Cancer 1984; 54: 2695–701. [DOI] [PubMed] [Google Scholar]
- 17. Jawien J. New insights into immunological aspects of atherosclerosis. Pol Arch Med Wewn 2008; 118: 127–31. [PubMed] [Google Scholar]
- 18. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Zoltan U. Vasculoprotective effects of anti‐tumor necrosis factor‐α treatment in aging. Am J Path 2007; 170: 388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Paola R, Mazzon E, Muià C et al . Effects of etanercept, a tumour necrosis factor‐α antagonist, in an experimental model of periodontitis in rats. Br J Pharmacol 2007; 150: 286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF‐κB. J Appl Physiol 2008; 105: 1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor‐α inhibition enhances NO modulation of vascular function in estrogen‐deficient rats. Hypertension 2005; 46: 76–81. [DOI] [PubMed] [Google Scholar]
- 22. Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft‐versus‐host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol 2007; 82: 45–52. [DOI] [PubMed] [Google Scholar]
- 23. Nair B, Raval G, Mehta P. TNF‐α inhibitor etanercept and hematologic malignancies. Report of a case and review of the literature. Am J Hematol 2007; 82: 1022–4. [DOI] [PubMed] [Google Scholar]
- 24. Ducasse E, Cosset JM, Eschwege F et al . External beam ionizing radiation for inhibition of myointimal hyperplasia after dilatation and anastomoses: experimental models and results. Ann Vasc Surg 2004; 18: 108–14. [DOI] [PubMed] [Google Scholar]


