Figure 5.
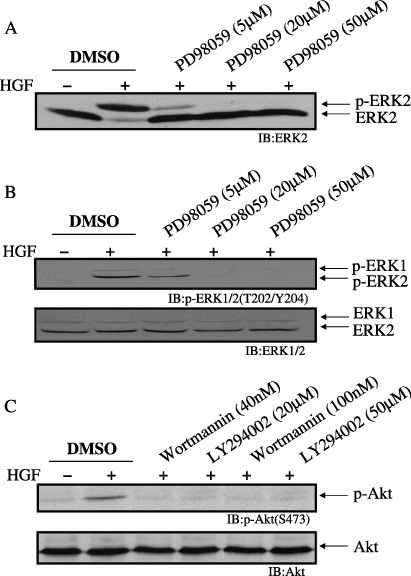
Effect of mitogen‐activated protein kinase/extracellular signal‐regulated kinase kinase (MEK)‐ or phosphoinositide 3‐kinase (PI3K)‐specific inhibitors on extracellular signal‐regulated kinase (ERK) phosphorylation and Akt (Ser473) phosphorylation. Porcine aortic endothelial (PAE) cells were pretreated for 1 h with vehicle (dimethylsulfoxide; DMSO), PD98059 (5 µM, 20 µM, 50 µM) (A and B), wortmannin (40 nM or 100 nM) or LY294002 (20 µM or 50 µM) (C), and treated for 5 min with (+) or without (–) hepatocyte growth factor (HGF) (50 ng/mL). Lysates of the cells were immunoblotted with an anti‐ERK2 antibody (A), an anti‐p‐ERK1/2 (T202/Y204) (B, upper panel) or anti‐ERK1/2 antibody (B, lower panel), an anti‐p‐Akt (S473) (C, upper panel) or anti‐Akt antibody (C, lower panel). (A) Phosphorylation of ERK2 proteins is indicated by a shift to a slower electrophoretic mobility. This mobility shift was not detected in (B), because the sodium dodecylsulfate–polyacrylamide gel electrophoresis system in (B) was different from that in (A).
