Abstract
Macrophage inflammatory protein 1α (MIP‐1α) is detected at high concentrations in patients with multiple myeloma, and it is thought to play an important role in the etiology of multiple myeloma and osteolysis. Thus, we investigated whether or not YM529/ONO‐5920, a new bisphosphonate, inhibited MIP‐1α mRNA expression in, and MIP‐1α secretion from, mouse myeloma cells. When the cells were stimulated by lipopolysaccharide, increased MIP‐1α mRNA expression and MIP‐1α secretion were observed. YM529/ONO‐5920 inhibited MIP‐1α mRNA expression and MIP‐1α secretion in a concentration‐dependent manner. A transient increase in the phosphorylation of extracellular‐regulated kinase 1/2 (ERK1/2) and Akt was observed after lipopolysaccharide stimulation. After YM529/ONO‐5920 was given, there was no transient increase in the phosphorylation of ERK1/2 or Akt. These results indicated that YM529/ONO‐5920 inhibited the expression and secretion of MIP‐1α through blocking the signaling pathway of the Ras/mitogen‐activated protein kinase kinase/ERK and Ras/phosphatidylinositol‐3 kinase/Akt. Accordingly, YM529/ONO‐5920 appears to have promise for use in effective future therapy for osteolysis and myeloma cell growth that depends on MIP‐1α. (Cancer Sci 2008; 99: 152–158)
Macrophage inflammatory protein 1α (MIP‐1α) is an osteoclast differentiation‐promoting factor.( 1 , 2 ) It is a chemokine of low molecular weight belonging to the regulated upon activation, normal T‐cell expressed and secreted family.( 3 ) As MIP‐1α is detected at high concentrations in bone marrow samples obtained from patients with advanced myeloma,( 2 , 3 , 4 , 5 ) it is thought to play an important role in the etiology of myeloma. According to several reports,( 2 , 4 , 6 , 7 ) the effects of MIP‐1α action include the secretion from tumor cells of interleukin‐6 (IL‐6) and parathyroid hormone‐related protein, which are osteoclast differentiation‐promoting factors, and the activation of osteoclasts by promoting the expression of cell adhesion factors. It has also been reported that MIP‐1α promotes the receptor activator of nuclear factor κB (NF‐κB) ligand expression of osteoblastic and interstitial cells and directly acts on osteoclasts to promote osteoclastogenesis and osteoclastic bone resorption.( 2 , 8 ) It is because of these actions that growth factors, such as insulin‐like growth factors and transforming growth factor α, are discharged from bone matrix into bone marrow, and the growth of tumor cells is accelerated if bone resorption increases. Moreover, it has also been reported that MIP‐1α is a tumor‐promoting factor because it accelerates its own growth with the help of autocrine from multiple myeloma (MM) cells.( 3 )
These reports suggest that a vicious circle takes place in which additional osteoclastic differentiation‐promoting factors are secreted, and osteoclastic bone resorption increases further.( 9 , 10 ) It is possible that osteoclastic activity might be inhibited through the use of a neutralization antibody to control MIP‐1α. It was confirmed that bone resorption was inhibited in SCID mice by inhibiting MIP‐1α with an antisense strand.( 6 ) Accordingly, it is feasible that the inhibition of MIP‐1α has the potential to serve as a therapeutic method for inhibiting the growth of tumor cells or preventing the destruction of bones. At present, however, there is no drug that can be used effectively to inhibit the secretion of MIP‐1α from tumor cells.
Bisphosphonate drugs are structural analogs of pyrophosphoric acid, and are used as therapeutic agents for osteoporosis and hypercalcemia. These drugs have a high affinity for bone minerals, so they accumulate in bone tissue after they have been taken, and they are thought to induce apoptosis in osteoclasts.( 11 ) Therefore, studies were conducted to determine the mechanism by which apoptosis is induced by nitrogen‐containing bisphosphonate drugs.( 12 ) It was found from these studies that bisphosphonate drugs inhibit farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) synthases in the mevalonic acid metabolic pathway, an intermediate metabolic pathway for the biosynthesis of cholesterol. As a result, it is known that bisphosphonate drugs inhibit FPP and GGPP synthesis and prevent the prenylation of low‐molecular‐weight G proteins, such as Ras, Rho, and Rac. It has been confirmed that YM529/ONO‐5920, a new bisphosphonate drug, causes the induction of apoptosis because this drug notably inhibits the biosynthesis of GGPP and the phosphorylation of extracellular‐regulated kinase (ERK) from among the mitogen‐activated protein kinases.( 13 )
If MIP‐1α released from myeloma cells can be inhibited by bisphosphonate drugs that act on not only osteoclasts, but also tumor cells, it would be possible to inhibit the growth of myeloma cells or prevent osteolysis. Therefore, the use of such bisphosphonate drugs might prolong lives, alleviate pain from osteolysis, or contribute to an improved quality of life of patients. Thus, we investigated whether or not there was any inhibitory effect on the MIP‐1α secreted from myeloma cells and the mechanism of any such inhibition.
Materials and Methods
Chemicals. YM529/ONO‐5920 (1‐hydroxy‐2‐(imidazo‐[1,2‐a]pyridin‐3‐yl) ethylidene bisphosphonic acid monohydrate) was supplied from Astellas Pharmaceutical (Tokyo, Japan). Lipopolysaccharide (LPS), IL‐6, and tumor necrosis factor α (TNF‐α) were purchased form Sigma (St. Louis, MO, USA). These reagents were dissolved in phosphate‐buffered saline (PBS; 0.05 M, pH 7.4), filtered through syringe filters (0.45 µm; Iwaki Glass, Tokyo, Japan) and used for various assays described below.
GGPP and FPP were purchased from Sigma. GGPP and FPP were dissolved in dry ethanol. Mitogen‐activated protein kinase kinase (MEK)1/2 inhibitor U0126 and phosphatidylinositol‐3 kinase (PI3K) inhibitor LY294002 were purchased from Promega (Madison, WI, USA) and dissolved in dimethylsulfoxide. These dissolved reagents were resuspended in PBS (0.05 M, pH 7.4) and filtered through syringe filters (0.45 µm; Iwaki Glass) before use.
Cell culture. We used mouse myeloma MOPC‐31C cells provided by Dainippon Pharmaceuticals (Osaka, Japan). Cells were cultured in L‐15 medium (Sigma) supplemented with 10% fetal bovine serum (Gibco, Rockville, MD, USA), penicillin (100 µg/mL; Meiji Seika Kaisha, Tokyo, Japan), streptomycin (100 units/mL; Meiji Seika Kaisha), and 25 mM HEPES (pH 7.4; Wako, Osaka, Japan) in an atmosphere containing 0% CO2.
Enzyme‐linked immunosorbent assay. MOPC‐31C cells were cultured at 1 × 104 cells/mL and conditioned media was harvested. The MIP‐1α levels were measured using Quantikine MIP‐1α enzyme immunoassay kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions.
Reverse transcription–polymerase chain reaction (RT‐PCR). Total RNA was extracted from the cultured MOPC‐31C cells using TRIzol (Invitrogen, Paisley, UK). Single‐stranded cDNA was synthesized from 1.0 µg of total RNA by incubating with oligo(dT)12–18 primer (Invitrogen) and SuperScript II RNase H‐reverse transcriptase (Invitrogen) using the SuperScript First‐Strand Synthesis System for RT‐PCR (Invitrogen).
cDNA was used as a template for PCR amplification to generate products corresponding to mRNA encoding the various gene products. Each PCR reaction contained cDNA, dNTPmix (Takara Biomedical, Siga, Japan), 10 × PCR buffer (Takara Biomedical), and Pyrobest (Takara Biomedical). DNA was amplified under the following typical cycling conditions: denaturation at 94°C for 0.5 min, annealing at 60°C for 0.5 min, and extension at 72°C for 0.5 min (30 cycles for glyceraldehyde‐3‐phosphate dehydrogenase); and denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min (35 cycles for MIP‐1α). PCR was carried out in a DNA thermal cycler (Takara Biomedical). The following primers were used: for MIP‐1α, 5′‐CAG CGA GTA CCA GTC CCT TTT‐′3 (5′‐primer) and 5′‐CCT CGC TGC CTC CAA GA‐3′ (3′‐primer); and for glyceraldehyde‐3‐phosphate dehydrogenase, 5′‐ACT TTG TCA AGC TCA TTT‐3′ (5′‐primer), 5′‐TGC AGC GAA CTT TAT TG‐3′ (3′‐primer). The PCR product was mixed with bromophenol blue‐loaded buffer and electrophoretically separated in a 2% agarose gel in Tris–acetate–EDTA buffer. After staining with ethidium bromide, the bands of PCR products were visualized with ultraviolet light and recorded with CoolSaver (ATTO, Tokyo, Japan).
Cell viability. Cells (1 × 103 cells/well) were plated in 96‐well plates and incubated with 1, 2, 5, 10, and 20 µM YM529/ONO‐5920. After incubation for 1 and 3 days, cells were stained with Trypan blue dye, and the number of stained cells was counted.
Western blotting. MOPC‐31C cells treated under various conditions were lyzed with a lysis buffer (20 mM Tris‐HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 100 mM NaF, 1% NP40, 1 µg/mL leupeptin, 1 µg/mL anti‐pain, 1 mM phenylmethylsulfonyl fluoride). The protein content of these cell lysates was determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Each extract (40 µg protein) was fractionated on a polyacrylamide–sodium dodecylsulfate gel, then transferred to a polyvinylidene difluoride membrane (Amersham Biosciences, Little Chalfont, UK). The membrane was incubated for 30 min for blocking in Tris‐buffered saline (10 mM Tris‐HCl (pH 7.4) and 150 mM NaCl) containing 3% skim milk, and incubated for 1 h at room temperature with each antibody (antiphospho‐ERK1/2 antibody, anti‐ERK1/2 antibody, antiphospho‐Akt antibody, anti‐Akt antibody, antiphospho‐p38 antibody, anti‐p38 antibody, and anti‐I‐κB antibody [Cell Signaling Technology, Beverly, MA, USA]) in Tris‐buffered saline. Subsequently, the membrane was incubated for 1 h at room temperature with antirabbit immunoglobulin G sheep antibody linked to horseradish peroxidase (Amersham Biosciences). Immunoreactive proteins were visualized by the ECL‐plus detection system (Amersham Biosciences).
Statistical analysis. Statistical analysis was carried out using the Mann–Whitney U‐test, and a value of P < 0.01 was considered statistically significant.
Results
Confirmation of MIP‐1α expression and secretion in MOPC‐31C cells. The amounts of MIP‐1α secreted from MOPC‐31C cells were examined 96 h after the addition of PBS(–) or LPS. At 96 h after the addition of LPS, MOPC‐31C cells secreted 110 pg/mL of MIP‐1α (Fig. 1a).
Figure 1.
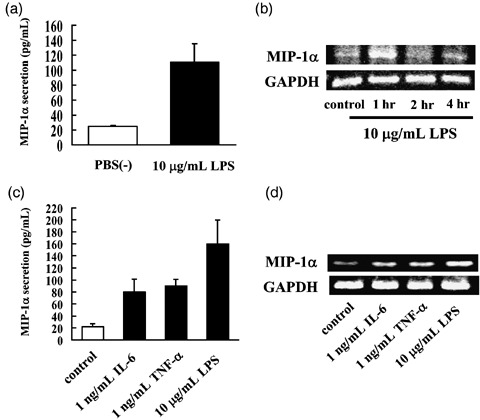
Confirmation of macrophage inflammatory protein 1α (MIP‐1α) secretion from MOPC‐31C mouse myeloma cells at the time of lipopolysaccharide (LPS) stimulation. (a) MOPC‐31C cells (1 × 103 cells/well) were incubated for 96 h with or without 10 µg/mL LPS. Culture supernatant was collected and analyzed for MIP‐1α by enzyme‐linked immunosorbent assay (ELISA). Data represent means ± standard deviations (SD) for at least quintuple samples. (b) Equal amounts of RNA were reverse transcribed to generate cDNA that was used for polymerase chain reaction (PCR) analysis of MIP‐1α mRNA expression in MOPC‐31C cells. (c) MOPC‐31C cells were incubated for 96 h with 1 ng/mL interleukin‐6 (IL‐6), 1 ng/mL tumor necrosis factor α (TNF‐α), or 10 µg/mL LPS. Culture supernatant was collected and analyzed for MIP‐1α by ELISA. Data represent means ± standard deviations for at least quintuple samples. (d) Equal amounts of RNA were reverse transcribed to generate cDNA that was used for PCR analysis of MIP‐1α mRNA expression in MOPC‐31C cells treated with IL‐6, TNF‐α, or LPS.
The expression of MIP‐1α mRNA was observed in MOPC‐31C cells of the PBC(–) group. When the cells were stimulated by LPS, increased expression of MIP‐1α mRNA was observed (Fig. 1b).
In MM cells, bone marrow stromal cell‐derived IL‐6 and TNF‐α are important factors for myeloma survival. In addition, it is known that IL‐6 and TNF‐α induce MIP‐1α secretion. Hence, we investigated whether IL‐6 and TNF‐α induce the expression of MIP‐1α mRNA and MIP‐1α secretion. Both IL‐6 and TNF‐α induced the expression of MIP‐1α mRNA and MIP‐1α secretion in MOPC‐31C cells (Fig. 1c,d). However, they induced MIP‐1α secretion at a lower level than LPS stimulation. Based on these results, we selected LPS as the stimulant of MIP‐1α secretion.
Determination of YM529/ONO‐5920 concentrations applied to MOPC‐31C cells. To evaluate the cytotoxic effects of YM529/ONO‐5920 on MOPC‐31C cells, the survival of these cells was assessed in the presence of YM529/ONO‐5920. We determined the cell survival rate, which was defined as the number of living cells at 1 and 3 days after receiving YM529/ONO‐5920 at concentrations of 1, 2, 5, 10, and 20 µM as compared with the number of live control cells treated with PBS. The survival rates at the respective concentrations were 106.5, 105.5, 102.0, 106.5 and 104.5% on day 1 and 97.0, 100.1, 98.2, 94.9, and 89.7% on day 3 (Fig. 2). As a significant decrease in the number of MOPC‐31C cells was observed at 3 days after 20 µM of YM529/ONO‐5920 was added, it was decided that YM529/ONO‐5920 should be added to MOPC‐31C cells at the concentration of 10 µM or lower, at which no cell death is induced.
Figure 2.
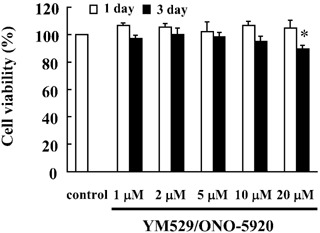
Determination of the YM529/ONO‐5920 concentrations added to mouse myeloma MOPC‐31C cells. Cells were incubated at a concentration of 1 × 104 cells/mL for 24 h in a 96‐well plate. These cells were treated with 1, 2, 5, 10, and 20 µM YM529/ONO‐5920. After incubation for 1 or 3 days, the number of viable cells was counted by means of Trypan blue staining, and the ratio of viable cells relative to those of the control was assessed as the cell survival rate. These results are representative of five independent experiments. *P < 0.01, compared to control.
Inhibitory effect of YM529/ONO‐5920 on MIP‐1α mRNA expression and MIP‐1α secretion in MOPC‐31C cells. YM529/ONO‐5920 inhibited the expression of MIP‐1α mRNA in a concentration‐dependent manner, and the inhibition was highest at the concentration of 10 µM (Fig. 3a). The inhibitory effect of YM529/ONO‐5920 on the secretion of MIP‐1α was examined in MOPC‐31C cells after the cells were stimulated by LPS. There was 106.96 pg/mL of MIP‐1α secretion when LPS was added to the cells. However, when both 10 µM YM529/ONO‐5920 and LPS were added to the cells, MIP‐1α secretion decreased to 30.76 pg/mL (Fig. 3b). Thus, the inhibition of MIP‐1α by YM529/ONO‐5920 was confirmed in a concentration‐dependent manner.
Figure 3.
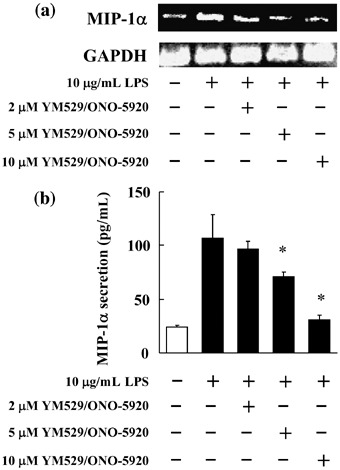
Inhibitory effect of YM529/ONO‐5920 on macrophage inflammatory protein 1α (MIP‐1α) mRNA expression and MIP‐1α secretion in MOPC‐31C mouse myeloma cells. (a) MOPC‐31C cells were treated with 2, 5, and 10 µM YM529/ONO‐5920. After incubation for 72 h, lipopolysaccharide (LPS) was added to give the final concentration of 10 µg/mL. After incubation for 1 h, RNA was extracted, and MIP‐1α mRNA expression was examined by reverse transcription–polymerase chain reaction (RT‐PCR). Photograph revealed the RT‐PCR analysis for MIP‐1α mRNA. (b) MOPC‐31C cells were treated with 2, 5, and 10 µM YM529/ONO‐5920 for 72 h. These cells receiving YM529/ONO‐5920 were cultured in the presence of LPS for 24 h. Culture supernatant was collected in culture and analyzed by enzyme‐linked immunosorbent assay. These results are representative of five independent experiments. *P < 0.01, compared to control.
Confirmation of the inhibitory effect of YM529/ONO‐5920 on expression of MIP‐1α mRNA in MOPC‐31C cells to which GGPP or FPP were also added. YM529/ONO‐5920 was combined with GGPP or FPP, products in the mevalonic acid pathway, to investigate whether or not the reduced MIP‐1α mRNA expression in MOPC‐31C cells was due to the inhibitory action of YM529/ONO‐5920 on GGPP or FPP biosynthesis exerted through its mechanism of action.
YM529/ONO‐5920 inhibited MIP‐1α mRNA expression, whereas in combination with GGPP, MIP‐1α mRNA expression was restored to the degree observed in the LPS group (Fig. 4a). This suggests that the inhibition of MIP‐1α mRNA expression in MOPC‐31C cells receiving YM529/ONO‐5920 was due to the inhibition of GGPP biosynthesis.
Figure 4.
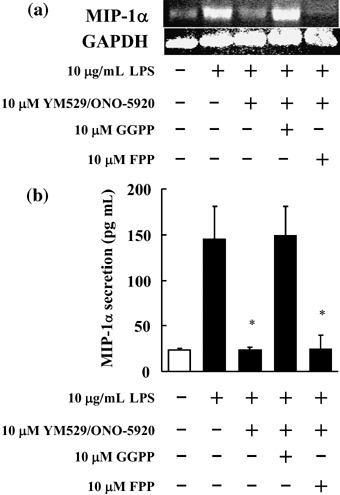
Macrophage inflammatory protein 1α (MIP‐1α) mRNA expression and protein secretion in MOPC‐31C mouse myeloma cells to which YM529/ONO‐5920 was added with or without geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP). (a) MOPC‐31C cells were pretreated with 10 µM GGPP or 10 µM FPP for 4 h then treated with 10 µM YM529/ONO‐5920 for 72 h. Photograph revealed the reverse transcription–polymerase chain reaction analysis of MIP‐1α mRNA expression. These cells receiving YM529/ONO‐5920 and GGPP or FPP were cultured in the presence of 10 µg/mL lipopolysaccharide (LPS) for 1 h. (b) MOPC‐31C cells were pretreated with 10 µM GGPP or 10 µM FPP for 4 h then treated with 10 µM YM529/ONO‐5920 for 72 h. After this treatment, MOPC‐31C cells were incubated for 24 h with 10 µg/mL LPS. Culture supernatant was collected and analyzed for MIP‐1α by enzyme‐linked immunosorbent assay. These results are representative of five independent experiments. *P < 0.01, compared to control.
A study was carried out to determine the effects of the previous addition of FPP or GGPP on the inhibitory effect that YM529/ONO‐5920 has on the secretion of MIP‐1α in MOPC‐31C cells after LPS stimulation. The amount of MIP‐1α secretion was 144.9 pg/mL in the control, but the corresponding amount was significantly inhibited to 23.5 pg/mL when YM529/ONO‐5920 was included. In the case of previous GGPP addition, the amount of secretion was 148.9 pg/mL (Fig. 4b).
Changes in phosphorylated ERK1/2, Akt, p38, and inhibitory protein I‐κB observed in MOPC‐31C cells at the time of LPS stimulation. Changes in phosphorylated ERK1/2, Akt, and p38 were studied to examine the signaling pathways used when LPS stimulation led to increases in the expression of MIP‐1α mRNA and in the secretion of protein observed in MOPC‐31C cells.
A transient increase in the phosphorylation of ERK1/2 was observed at 5 and 15 min after LPS stimulation, as compared to the control. In the phosphorylation of Akt, a transient increase was observed at 30 and 60 min after LPS stimulation, as compared to the control. The activation of NF‐κB was examined as the decomposition of I‐κB protein. A remarkable decrease in the I‐κB level was also observed at 15 min after LPS stimulation (Fig. 5a).
Figure 5.
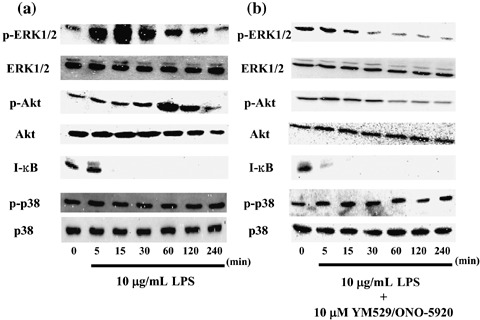
Time‐course changes in phosphorylated extracellular‐regulated kinase (ERK), Akt, p38, and I‐κB proteins observed in MOPC‐31C mouse myeloma cells at the time of lipopolysaccharide (LPS) stimulation with or without YM529/ONO‐5920 pretreatment. (a) MOPC‐31C cells were cultured in the presence of 10 µg/mL LPS for 5, 15, 30, 60, 120, and 240 min. Whole cell lysates were generated and immunoblotted with an antibody against phosphorylated ERK1/2 (p‐ERK1/2), phosphorylated Akt (p‐Akt), phosphorylated p38 (p‐p38), ERK1/2, Akt, I‐κB, and p38. (b) MOPC‐31C cells were treated with 10 µM YM529/ONO‐5920 for 72 h. These cells receiving YM529/ONO‐5920 were cultured in the presence of 10 µg/mL LPS.
It was suggested that a signaling pathway by way of ERK1/2, Akt, and NF‐κB is involved in the expression and secretion of MIP‐1α observed in MOPC‐31C cells at the time of LPS stimulation.
The addition of YM529/ONO‐5920 gave no transient increase in the phosphorylation of ERK1/2 after the addition of LPS. On the contrary, this protein showed a decrease in phosphorylation at 60, 120, and 240 min after the LPS stimulation. After the addition of YM529/ONO‐5920, there was no transient increase in the phosphorylation of Akt, and a decrease from the control level was confirmed at 60, 120, and 240 min after LPS stimulation. However, a decrease in I‐κB level by LPS stimulation was observed 5 min after YM529/ONO‐5920 was added (Fig. 5b).
Inhibited expression of MIP‐1α mRNA and inhibited secretion of MIP‐1α protein resulting from the addition of U0126 and LY294002. The findings suggested that MIP‐1α expression is inhibited in MOPC‐31C cells by inhibiting GGPP biosynthesis in the mevalonic acid pathway, thereby blocking the signaling pathway through intermediation of the Ras/MEK/ERK pathway. Therefore, we attempted to confirm whether MIP‐1α expression is inhibited by inhibiting ERK1/2. U0126, a MEK1/2 inhibitor, was added to MOPC‐31C cells and the consequent effect on MIP‐1α mRNA expression and MIP‐1α secretion was examined.
As a result, it was confirmed that, like YM529/ONO‐5920, the addition of U0126 inhibited the expression of MIP‐1α mRNA (Fig. 6a). The amount of MIP‐1α secreted was 19.2 pg/mL, compared to 144.9 pg/mL obtained with LPS stimulation. U0126 therefore appeared to inhibit MIP‐1α secretion to the same level as YM529/ONO‐5920 (Fig. 6b). In addition, we observed that U0126 suppressed ERK phosphorylation following LPS stimulation (Fig. 6c). These results suggested that the production of MIP‐1α is inhibited by blocking the signaling pathway through intermediation of the MEK/ERK pathway.
Figure 6.
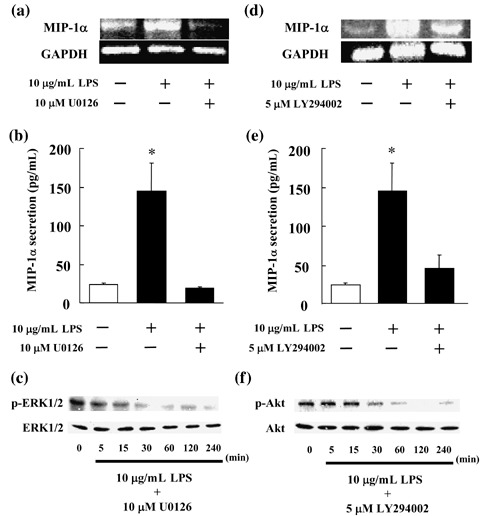
Inhibition of macrophage inflammatory protein 1α (MIP‐1α) mRNA expression and protein secretion in MOPC‐31C mouse myeloma cells treated with U0126 (a mitogen‐activated protein kinase kinase 1/2 inhibitor) or LY294002 (a phosphatidylinositol‐3 kinase inhibitor). (a, d) MOPC‐31C cells were treated with 10 µM U0126 or 5 µM LY294002 for 72 h. These cells were cultured in the presence of 10 µg/mL lipopolysaccharide (LPS) for 1 h. Photograph revealed the reverse transcription–polymerase chain reaction analysis for MIP‐1α. (b, e) MOPC‐31C cells were treated with 10 µM U0126 or 5 µM LY294002 for 72 h. These cells receiving U0126 or LY294002 were cultured in the presence of 10 µg/mL LPS for 24 h. Culture supernatant was collected and analyzed by enzyme‐linked immunosorbent assay. These results are representative of five independent experiments. *P < 0.01, compared to control. (c, f) MOPC‐31C cells were treated with 10 µM U0126 or 5 µM LY294002 for 72 h. These treated cells were cultured in the presence of 10 µg/mL LPS for 5, 15, 30, 60, 120, and 240 min. Whole cell lysates were prepared and immunoblotted with antibodies against phosphorylated ERK1/2 (p‐ERK1/2), phosphorylated Akt (p‐Akt), ERK1/2, and Akt.
Concerning the changes in proteins observed in MOPC‐31C cells at the time of LPS stimulation, it was confirmed that the amount of Akt increased at 30 and 60 min after LPS stimulation. Therefore, LY294002 was added to MOPC‐31C cells to determine whether or not the production of MIP‐1α is inhibited by inhibiting Akt, and if LY294002 affects the expression of MIP‐1α mRNA and the secretion of MIP‐1α. The expression of MIP‐1α mRNA was inhibited by the addition of LY294002, although the rate of inhibition was lower than that of YM529/ONO‐5920 (Fig. 6d). The amount of MIP‐1α secreted was 45.33 pg/mL, as compared to the 144.9 pg/mL of MIP‐1α secreted with the addition of LPS (Fig. 6e). Furthermore, we observed that LY294002 suppressed Akt phosphorylation following LPS stimulation (Fig. 6f). Thus, inhibition of MIP‐1α secretion was confirmed. These results indicated that the production of MIP‐1α is inhibited by blocking the signaling pathway through the intermediation of the PI3K/Akt pathway.
Inhibitory effect of YM529/ONO‐5920 on MIP‐1α‐dependent MOPC‐31C cell growth. We also investigated whether YM529/ONO‐5920 inhibited MIP‐1α‐dependent MOPC‐31C cell growth. LPS stimulated MOPC‐31C cell growth, whereas MIP‐1α neutralizing antibody suppressed the cell growth after LPS was added (Fig. 7a). Thus, the suppression of MIP‐1α inhibits MOPC‐31C cell growth.
Figure 7.
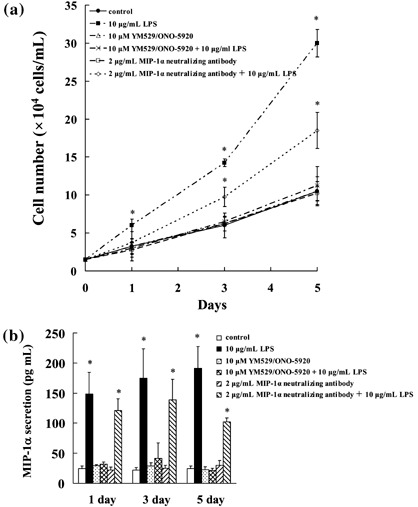
Inhibitory effect of YM529/ONO‐5920 on macrophage inflammatory protein 1α (MIP‐1α)‐dependent MOPC‐31C mouse myeloma cell growth. (a) Cells were incubated at a concentration of 1 × 104 cells/mL for 24 h in a 96‐well plate. These cells were treated with 10 µg/mL LPS, 10 µM YM529/ONO‐5920, 10 µM YM529 + 10 µg/mL LPS, 2 µg/mL MIP‐1α neutralizing antibody, or 2 µg/mL MIP‐1α neutralizing antibody + 10 µg/mL LPS. After incubation for 1, 3, or 5 days, the number of viable cells was counted by Trypan blue staining. The results are representative of five independent experiments. (b) MOPC‐31C cells were treated with 10 µg/mL LPS, 10 µM YM529/ONO‐5920, 10 µM YM529 + 10 µg/mL LPS, 2 µg/mL MIP‐1α neutralizing antibody, or 2 µg/mL MIP‐1α neutralizing antibody + 10 µg/mL LPS. After incubation for 1, 3, or 5 days, culture supernatant was collected and analyzed by enzyme‐linked immunosorbent assay. These results are representative of five independent experiments. *P < 0.01, compared to control.
YM529/ONO‐5920 inhibited MOPC‐31C cell growth on LPS stimulation by suppressing MIP‐1α secretion (Fig. 7a,b). These results suggest that YM529/ONO‐5920 inhibited MIP‐1α‐dependent MOPC‐31C cell growth.
Discussion
MIP‐1α is an important factor produced at high levels in myeloma patients.( 5 ) It promotes osteoclastogenesis, accelerates osteoclastic bone resorption, and develops osteolytic lesions. These actions derive from the secretion of osteoclastic differentiation‐promoting factors from tumor cells, the promotion of receptor activator of NF‐κB ligand expression of osteoblastic and interstitial cells, and direct action on osteoclasts.( 8 ) It has also been reported that MIP‐1α is a tumor‐promoting factor because it accelerates its own growth with the help of autocrine from MM cells.( 3 ) Therefore, drugs that inhibit the production of MIP‐1α are expected to inhibit the destruction of bone and the growth of tumors in MM cells, and have the potential to serve as very effective therapeutic agents.
In this experiment, our group found that third‐generation bisphophonate drugs inhibited MIP‐1α secreted from MM cells. It is reported that these bisphosphonate drugs, which are therapeutic drugs for osteoporosis, serve to inhibit prenylation of G proteins, such as Ras and Rho, by inhibiting the production of FPP and GGPP. Incadronate and YM529/ONO‐5920 are also bisphosphonate drugs, and are reported to inhibit the mevalonic acid and mitogen‐activated protein kinase pathways and thereby to induce apoptosis in myeloma cells. Reportedly, the mechanism of action is inhibition of GGPP biosynthesis.( 14 )
In the present experiment, the inhibitory effect of YM529/ONO‐5920 on MIP‐1α secretion was negated by the previous addition of GGPP, but not by the previous addition of FPP. Our results and those of other reports together suggest that the inhibition of MIP‐1α secretion by the addition of YM529/ONO‐5920 is attributable to the inhibition of GGPP synthesis.
The treatment of MOPC‐31C cells with 10 µM YM529/ONO‐5920 for 3 days in vitro inhibited GGPP synthesis. The peak plasma concentrations of bisphosphonate drugs achieved with standard doses were = 10 µM.( 15 , 16 ) Moreover, in the bone, the local concentrations of bisphosphonate drugs have been calculated in the range of 0.1–1.0 mM.( 17 ) These findings indicate that the 10 µM concentration of YM529/ONO‐5920 is within the peak plasma and bone concentrations of YM529/ONO‐5920 that are likely to be achieved in vivo.
LPS is known to combine with a protein family called toll‐like receptors (TLRs) and to activate TLR1, 2, 4, and 9.( 18 ) After combination with TLRs, a complex of TLR–MyD88–IL‐1 receptor‐associated kinase is formed in the cells. At that time, NF‐κB, a transcription factor necessary for the transcription of cytokines, is activated in order to induce the activation of a target gene. This complex is also reported to induce the activation of the Ras pathway and I‐κB kinases by way of TNF receptor‐associated factor 6.( 19 , 20 ) These reports suggest that Ras and NF‐κB are involved in the production of MIP‐1α. It has been reported that the production of MIP‐1α induced by LPS and TNF‐α is inhibited by inhibiting NF‐κB in human macrophages.( 21 ) However, there has been no report on functioning of the signaling pathway in the production of MIP‐1α caused by LPS stimulation in myeloma cells. In the present experiment, the activation of NF‐κB was observed when LPS was added to MOPC‐31C cells. At the same time, a transient activation of ERK1/2 and Akt was also observed. The combination of YM529/ONO‐5920 + LPS did not affect the activation of NF‐κB, but phosphorylation of ERK1/2 and Akt was inhibited, and subsequently, decreases in the expression of MIP‐1α mRNA and the secretion of MIP‐1α were confirmed. These results suggest that the signaling of ERK1/2 and Akt is important for the production of MIP‐1α.
A study was conducted with U0126 to determine whether or not ERK1/2 inhibition actually inhibits MIP‐1α secretion. When U0126 was added, it was confirmed that, like YM529/ONO‐5920, U0126 inhibited MIP‐1α expression and ERK1/2 activity. LY294002, a PI3K inhibitor, was also used in the study to confirm whether or not the inhibition of Akt leads to the inhibition of MIP‐1α secretion. It was confirmed that LY294002 also inhibited the expression of MIP‐1α and suppressed the activity of Akt, similar to the effect of YM529/ONO‐5920.
These results suggested that the inhibitory effect of YM529/ONO‐5920 on the secretion of MIP‐1α in MOPC‐31C cells is brought out by the inhibition of GGPP biosynthesis, which in turn inhibits the activation of the Ras/MEK/ERK and Ras/PI3K/Akt pathways.
As described above, YM529/ONO‐5920 is known to affect the functions of Ras by inhibiting prenylation through the inhibition of GGPP synthesis; this enables localization of Ras at the plasma membrane.( 22 ) Ras is involved in the activation of the MEK/ERK and PI3K/Akt pathways,( 23 , 24 ) suggesting the mechanism of action of YM529/ONO‐5920.
Activation of the Ras/MEK/ERK pathway was thought to be essential in the MIP‐1α secretion because an increase in the phosphorylation of ERK1/2 was observed in the promotion of MIP‐1α expression and secretion caused by adding LPS and the promotion of MIP‐1α expression and secretion were completely inhibited by U0126, a MEK1/2 inhibitor. Although the details remain obscure, there might be some involvement of the Ras/PI3K/Akt pathway because LY294002 inhibited activation of Akt at the time of LPS stimulation and MIP‐1α expression decreased along with the inhibited activation of Akt to a lesser extent than when the MEK/ERK pathway was inhibited by U0126.
In this experiment, YM529/ONO‐5920 inhibited MOPC‐31C cell growth induced by LPS stimulation. At this stage, YM529/ONO‐5920 suppressed MIP‐1α secretion induced by LPS stimulation. These results suggest that YM529/ONO‐5920 inhibited MIP‐1α‐dependent MOPC‐31C cell growth.
In the present experiment, the inhibitory effect of YM529/ONO‐5920 on the secretion of MIP‐1α was confirmed in the MM cells. Accordingly, bisphosphonate drugs with the property of high bone accumulation appear to have promise for use in effective future therapy for osteolysis and myeloma cell growth that depend on MIP‐1α secreted from myeloma cells.
Acknowledgments
This work was supported by the ‘High‐Tech Research Center’ project for private universities, Japan, matching a funding subsidy from the Ministry of Education, Culture, Sports, Science and Technology of Japan, 2007–2011.
References
- 1. Yu X, Huang Y, Collin‐Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res 2004; 19: 2065–77. [DOI] [PubMed] [Google Scholar]
- 2. Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein‐1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood 2001; 97: 3349–53. [DOI] [PubMed] [Google Scholar]
- 3. Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1‐alpha (MIP‐1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003; 101: 3568–73. [DOI] [PubMed] [Google Scholar]
- 4. Choi SJ, Cruz JC, Craig F et al . Macrophage inflammatory protein 1‐alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood 2000; 96: 671–5. [PubMed] [Google Scholar]
- 5. Terpos E, Politou M, Szydlo R, Goldman JM, Apperley JF, Rahemtulla A. Serum levels of macrophage inflammatory protein‐1 alpha (MIP‐1alpha) correlate with the extent of bone disease and survival in patients with multiple myeloma. Br J Haematol 2003; 123: 106–9. [DOI] [PubMed] [Google Scholar]
- 6. Choi SJ, Oba Y, Gazitt Y et al . Antisense inhibition of macrophage inflammatory protein 1‐alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest 2001; 108: 1833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oba Y, Lee JW, Ehrlich LA et al . MIP‐1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp Hematol 2005; 33: 272–8. [DOI] [PubMed] [Google Scholar]
- 8. Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res 2002; 17: 1921–5. [DOI] [PubMed] [Google Scholar]
- 9. Winding B, Misander H, Sveigaard C et al . Human breast cancer cells induced angiogenesis, recruitment, and activation of osteoclasts in osteolytic metastasis. J Cancer Res Clin Oncol 2000; 126: 631–40. [DOI] [PubMed] [Google Scholar]
- 10. Guise TA, Mundy GR. Cancer and bone. Endocr Rev 1998; 19: 18–54. [DOI] [PubMed] [Google Scholar]
- 11. Fleisch H. Bisphosphonate: mechanisms of action. Endocr Rev 1998; 19: 80–100. [DOI] [PubMed] [Google Scholar]
- 12. Shipman CM, Croucher PI, Graham R, Russell G, Helfrich MH, Rogers MJ. The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res 1998; 58: 5294–7. [PubMed] [Google Scholar]
- 13. Nishida S, Fujii Y, Yoshioka S, Kikuichi S, Tsubaki M, Irimajiri K. A new bisphosphonate, YM529 induces apoptosis in HL60 cells by decreasing phosphorylation of single survival signal ERK. Life Sci 2003; 73: 2655–64. [DOI] [PubMed] [Google Scholar]
- 14. Iguchi T, Miyakawa Y, Yamamoto K, Kizaki M, Ikeda Y. Nitrogen‐containing bisphosphonates induce S‐phase cell cycle arrest and apoptosis of myeloma cells by activating MAPK pathway and inhibiting mevalonate pathway. Cell Signal 2003; 15: 719–27. [DOI] [PubMed] [Google Scholar]
- 15. Yamagishi S, Abe R, Inagaki Y et al . Minodronate, a newly developed nitrogen‐containing bisphosphonate, suppresses melanoma growth and improves survival in nude mice by blocking vascular endothelial growth factor signaling. Am J Pathol 2004; 165: 1865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon S, Helfrich MH, Sati HI et al . Pamidronate causes apoptosis of plasma cells in vivo in patients with multiple myeloma. Br J Haematol 2002; 119: 475–83. [DOI] [PubMed] [Google Scholar]
- 17. Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer‐induced bone disease: beyond their antiresorptive activity. Cancer Res 2005; 65: 4971–4. [DOI] [PubMed] [Google Scholar]
- 18. Akira S, Takeda K. Toll‐like receptor signaling. Nat Rev Immunol 2004; 4: 499–511. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK‐M is a negative regulator of toll‐like receptor signaling. Cell 2002; 110: 191–202. [DOI] [PubMed] [Google Scholar]
- 20. Xu H, An H, Yu Y, Zhang M, Qi R, Cao X. Ras participates in CpG oligodeoxynucleotide signaling through association with toll‐like receptor 9 and promotion of interleukin‐1 receptor‐associated kinase/tumor necrosis factor receptor‐associated factor 6 complex formation in macrophages. J Biol Chem 2003; 278: 36334–40. [DOI] [PubMed] [Google Scholar]
- 21. Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. TNFalpha‐induced macrophage chemokine secretion is more dependent on NF‐kappaB expression than lipopolysaccharides‐induced macrophage chemokine secretion. Eur J Immunol 2002; 32: 2037–45. [DOI] [PubMed] [Google Scholar]
- 22. Segawa H, Kimura S, Kuroda J et al . The anti‐leukemic efficacy of the third generation bisphosphonate ONO5920/YM529. Leuk Res 2005; 29: 451–7. [DOI] [PubMed] [Google Scholar]
- 23. Caraglia M, Santini D, Marra M, Vincenzi B, Tonini G, Budillon A. Emerging anti‐cancer molecular mechanisms of aminobisphosphonates. Endocr Relat Cancer 2006; 13: 7–26. [DOI] [PubMed] [Google Scholar]
- 24. Hu L, Shi Y, Hsu JH, Gera J, Van Ness B, Lichtenstein A. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood 2003; 101: 3126–35. [DOI] [PubMed] [Google Scholar]


