Figure 2.
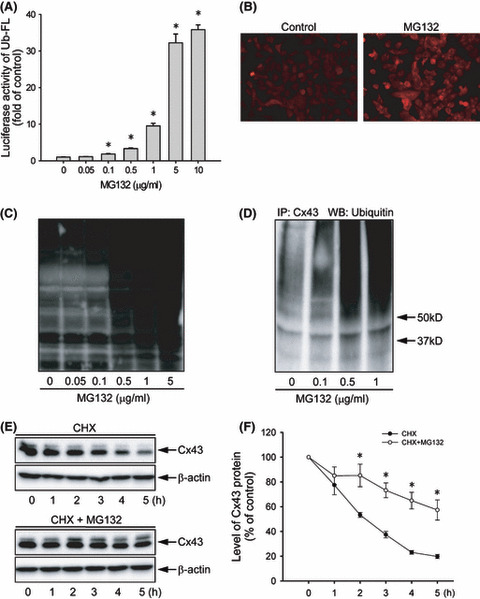
Effect of MG132 on proteasome function and connexin 43 (Cx43) degradation. (A) Effect of MG132 on ubiquitin–luciferase bioluminescence imaging reporter (Ub‐FL) activity. Hepa‐1c1c7 cells were transiently transfected with a Ub‐FL reporter and exposed to the indicated concentrations of MG132. The relative luciferase activity is expressed as fold induction over untreated control (mean ± SE, n = 4). *P < 0.01 versus untreated control. (B) Immunofluorescent staining for ubiquitin. Hepa‐1c1c7 cells were either left untreated or incubated with 0.5 μg/mL MG132 for 12 h, and then subjected to immunofluorescent staining of ubiqutin (red). Note the obvious enhanced intensity of ubiqutin (red) at the perinuclear region. Magnification, ×400. (C) Effect of MG132 on protein ubiqutiylation. Hepa‐1c1c7 cells were exposed to various concentrations of MG132 for 12 h. The cellular protein was extracted and subjected to western blotting analysis of ubiquitylated proteins. (D) Effect of MG132 on ubiquitylation of Cx43. Hepa‐1c1c7 cells were treated with the indicated concentrations of MG132 for 12 h. Cell lysates were subjected to immunoprecipitation (IP) with an anti‐Cx43 antibody and blotted with an anti‐ubiquitin antibody. (E) Effect of MG132 on Cx43 protein degradation. Hepa‐1c1c7 cells were exposed to 50 μg/mL cycloheximide (CHX) in the presence or absence of 0.5 μg/mL MG132 for the indicated times. Cellular proteins were analyzed by western blotting (WB) with an anti‐Cx43 antibody. A representative blot is shown in (E). (F) The intensity of each Cx43 signal in (E) was measured and the relative intensity of the band against its intensity at zero point are shown (mean ± SE, n = 4). *P < 0.01 versus untreated control.
