Figure 3.
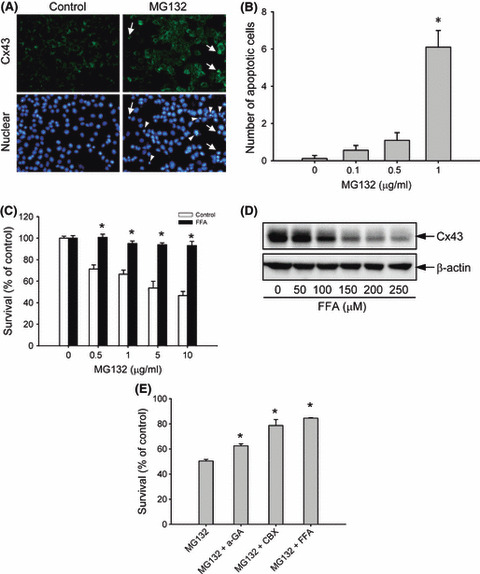
Involvement of gap junctions in MG132‐induced cell damage. (A) Concomitant induction of connexin 43 (Cx43) and cell apoptosis by MG132. Hepa‐1c1c7 cells were either left untreated or incubated with 1 μg/mL MG132 for 12 h, and then subjected to immunofluorescence staining of Cx43 (green; upper panel) and nuclei (DAPI stain, blue; lower panel). Arrow and arrow heads indicate apoptotic cells. Note the coexistence of elevated Cx43 and nuclear condensation in part of the indicated cells. Magnification, ×400. (B) The number of apoptotic cells after MG132 treatment. The cells with nuclear condensation and fragmentation were quantified and expressed as apoptotic cells per field (mean ± SE, n = 10). *P < 0.01 versus respective control. (C) Effect of the gap junction inhibitor flufenamic acid (FFA) on MG132‐elicited loss of cell viability. Hepa‐1c1c7 cells were exposed to the indicated concentrations of MG132 in the presence or absence of 150 μm FFA for 12 h. Cellular viability was determined by formazan assay. The data are expressed as a percentage of the control (mean ± SE, n = 4). *P < 0.01 versus respective control. (D) Effect of FFA on Cx43 protein levels. 1c1c‐7 cells were exposed to the indicated concentrations of FFA for 12 h. The cellular protein was extracted and subjected to western blot analysis of Cx43. Expression of β‐actin is shown at the bottom as a loading control. (E) Effects of several different gap junction inhibitors on MG132‐elicited loss of cell viability. Hepa‐1c1c7 cells were exposed to 5 μg/mL MG132 in the presence or absence of 10 μm 18‐α glycyrrhetinic (α‐GA), 10 μm carbenoxolone (CBX), or 150 μm FFA for 12 h. Cellular viability was determined by formazan assay. The data are expressed as a percentage of the control (mean ± SE, n = 4). *P < 0.01 versus MG132 alone.
