Figure 1.
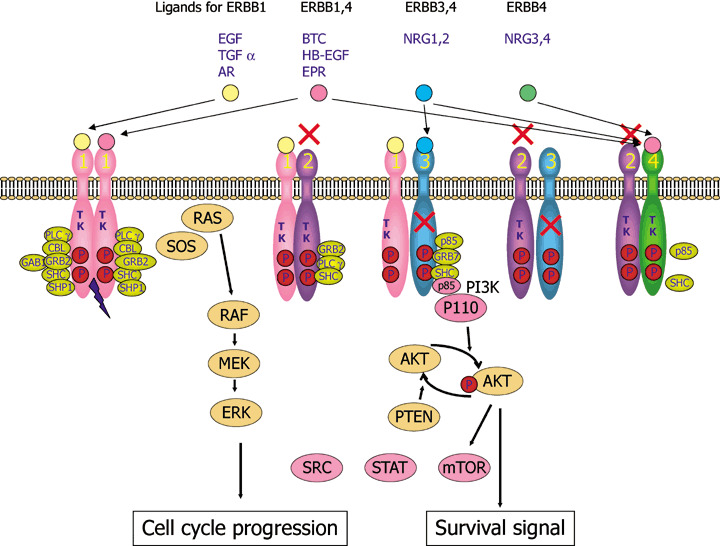
ERBB signaling pathways. Binding of a family of specific ligands to extracellular domain of ERBB leads to formation of homo‐ and heterodimers. In this case, HER2 is a preferred dimerization partner and heterodimers containing HER2 mediate a stronger signal than homodimers. Dimerization consequently stimulates intrinsic tyrosine kinase activity of the receptors and triggers autophosphorylation of specific tyrosine residues within the cytoplasmic domain. These phosphorylated tyrosines serve as specific binding sites for several signal transducers that initiate multiple signaling pathways including mitogen‐activated protein kinase (MAPK), phosphatidyl inositol 3 kinase (PI3K)‐AKT and signal transducer and activator of transcription protein (STAT) 3 and 5 pathways. These eventually result in cell proliferation, migration and metastasis, evasion from apoptosis, or angiogenesis, all of which are associated with cancer. P85 and p110 is a regulatory and catalytic subunit of phosphatidyl inositol 3 kinase (PI3K), respectively. STAT, SRC and mTOR are also activated by ERBB sinaling. AR, amphiregulin; BTC, betacellulin; EPR, epirefulin; ERK, extracellular signal‐regulated kinase; HB‐EGFR, heparin binding EGF; MEK, MAP an ERK kinase; mTOR, mammmalian target of rapamycin; NRG, neuregulin; TGF, transforming growth factor.
