Figure 1.
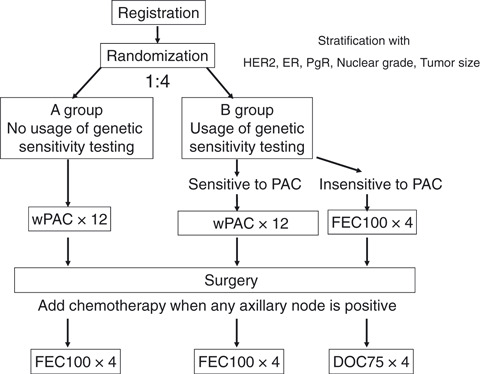
Study design. Patients were stratified according to the status of human epidermal growth factor receptor type 2 (HER2), estrogen receptor (ER), progesteron receptor (PgR), nuclear grade and tumor size. Patients were randomly assigned to receive arm A or B with a ratio of 1:4. In arm A, patients received primary chemotherapy with paclitaxel without selection by genetic sensitivity testing. For patients in arm B, we used the genetic diagnosis for sensitivity to paclitaxel. In arm B1, patients diagnosed as sensitive to paclitaxel received paclitaxel. In arm B2, patients diagnosed as insensitive to paclitaxel received FEC100. When any axillary node was positive for cancer after curative breast surgery, additional chemotherapy was used. Patients pretreated with paclitaxel received FEC100. Patients pretreated with FEC100 received docetaxel. wPAC × 12, 12 cycles of weekly paclitaxel 80 mg/m2; FEC100 × 4, four cycles of combination chemotherapy with fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cycrophosphamide 500 mg/m2 every 3 weeks; DOC75 × 4, four cycles of docetaxel 75 mg/m2 every 3 weeks.
