Figure 3.
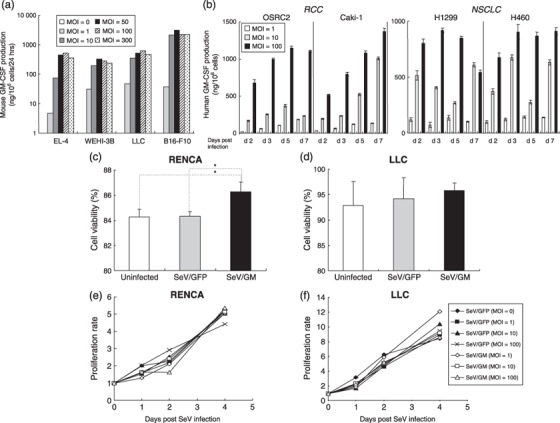
Granulocyte macrophage colony‐stimulating factor (GM‐CSF) production from mouse or human tumor cell lines transduced with SeV/dF/mGM or SeV/dF/hGM, and the viability and proliferation of Sendai virus (SeV)‐transduced cells. (a) One million cells from four mouse tumor cell lines were transduced with SeV/dF/mGM at multiplicities of infection (MOI) of 0, 1,10, 50, 100, and 300 for 90 min in serum‐free RPMI‐1640 and incubated for 10% fetal bovine serum (FBS)/RPMI in 6‐well plates for 24 h. Mouse GM‐CSF levels produced in each supernatant were measured by enzyme‐linked immunosorbent assays. (b) Human GM‐CSF levels produced by four human cell lines (two for non‐small‐cell lung cancer [NSCLC] and two for renal cell carcinoma [RCC]) transduced with SeV/dF/hGM at MOI of 1, 10, and 100 on days 2, 3, 5, and 7 after transduction were measured by enzyme‐linked immunosorbent assays. Cell viability after SeV infection was evaluated by trypan blue exclusion. (c,d) Two million parental RENCA or Lewis lung carcinoma (LLC) cells were transduced with SeV/dF/GFP (MOI = 100) or SeV/dF/mGM (MOI = 100) for 90 min, and cultured for 48 h. The number of trypan blue‐positive and ‐negative cells was counted under a light microscope, and the percentage of cells excluding trypan blue is represented as an index of cell viability. (e,f) RENCA and LLC cells were cultured separately in 96‐well microplates at 1 × 104 cells/well. They were transduced with SeV/dF/GFP (MOI = 1, 10, or 100) or SeV/dF/mGM (MOI = 1, 10, or 100) for 90 min in serum‐free medium, and cultured for 1, 2, and 4 days in RPMI‐1640 with 10% FBS or Dulbecco's modified Eagle's medium with 10% FBS, respectively. At each time point (day 0, 1, 2, or 4 after SeV transduction), the number of viable cells was estimated spectrophotometrically by the incorporation of tetrazolium dye. Representative data from three independent experiments are shown.
