Abstract
Heat shock protein (Hsp) 90 is a key regulator of a variety of oncogene products and cell‐signaling molecules, and the therapeutic benefit of its inhibition in combination with radiation or chemotherapy has been investigated. In addition, hyperthermia has been used for many years to treat various malignant tumors. We previously described a system in which hyperthermia was induced using thermosensitive ferromagnetic particles (FMP) with a Curie temperature (Tc = 43˚C) low enough to mediate automatic temperature control, and demonstrated its antitumor effect in a mouse melanoma model. In the present study, we examined the antitumor effects of combining a Hsp90 inhibitor (geldanamycin; GA) with FMP‐mediated hyperthermia. In cultured B16 melanoma cells, GA exerted an antitumor effect by increasing the cells’ susceptibility to hyperthermia and reducing expression of Akt. In an in vivo study, melanoma cells were subcutaneously injected into the backs of C57BL/6 mice. FMP were then injected into the resultant tumors, and the mice were divided into four groups: group I, no treatment (control); group II, one hyperthermia treatment; group III, GA alone; and group IV, GA with hyperthermia. When exposed to a magnetic field, the temperature of tissues containing FMP increased and stabilized at the Tc. In group IV, complete regression of tumors was observed in five of nine mice (56%), whereas no tumor regression was seen in groups I–III. Our findings suggest that inhibition of Hsp90 with hyperthermia increases its antitumor effect. Thus, the combination of FMP‐mediated, self‐regulating hyperthermia with Hsp90 inhibition has important implications for the treatment of cancer. (Cancer Sci 2009; 100: 558–564)
Hyperthermia is used in the treatment of tumors because tumor cells are more sensitive to temperature in the range of 42–45°C than are normal tissue cells.( 1 , 2 , 3 , 4 ) Still higher temperatures (up to 56°C) lead to widespread necrosis, coagulation, or carbonization in a process called ‘thermoablation’. Hyperthermia has an advantage over thermoablation in that it has fewer side effects, and its use, alone and in combination with chemotherapy or radiation, in the treatment of a wide variety of malignant tumors has been investigated in both experimental animals and in patients.( 5 ) The most commonly used method of heating in clinical settings is capacitive heating using a radiofrequency electrical field.( 4 , 6 , 7 ) The great advantage of capacitive heating is that it is non‐invasive. However, this method can cause excessive heating of the fat layer and is not suitable for site‐specific hyperthermia because it is difficult to selectively heat only the local tumor region to the intended temperature without also damaging normal tissue. This is because as the electrical field energy is conducted through the normal tissue, it is imperfectly transduced to heat in a manner reflecting the specific rates of absorption by the tissues, which are dependent on their specific electrical properties (e.g. permittivity and resistance).
To overcome the disadvantages of capacitive heating for the treatment of tumors, attempts have been made to use inductive heating with magnetic nanoparticles.( 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ) When placed within a magnetic field, ferromagnetic materials develop an electrical current and generate heat due to hysteresis loss.( 18 ) Heating through magnetic induction using a thermosensitive material is able to produce highly localized hyperthermia in deep‐seated tumors. However, precise control of the temperature has proven to be a difficult and complicated challenge. To address that challenge, we developed thermosensitive ferromagnetic particles (FMP) that produce sufficient amounts of heat through production of eddy currents, and to have a Curie temperature (Tc) low enough to mediate self‐regulated temperature control. The Tc is a transition point at which a material loses its magnetic properties, which causes current flow, and thus heat production, to cease.( 19 , 20 ) A low Tc (43°C) can enable automatic temperature control throughout a tumor because the self‐regulating nature of the thermosensitive materials will correct for local variations in heat loss due to blood perfusion. Moreover, because the average diameter of our FMP is approximately 100 µm, they can be directly injected into any tumor site, either percutaneously or using a fiberscope (e.g. for tracheal or gastrointestinal tumors), after which the FMP will remain at the injected site because they are larger than blood capillaries and lymphatic vessels.( 21 ) This feature makes this method minimally invasive, as it is possible to administer repeated hyperthermia treatments after a single injection. In an earlier experiment, we used a mouse melanoma model to demonstrate the antitumor effect of self‐regulating, FMP‐mediated hyperthermia.( 22 )
The heat shock protein (Hsp) family includes a group of molecular chaperones that appear to play important roles during protein folding( 23 ) and conformational maturation.( 24 , 25 ) Among those, Hsp90 is crucially involved in the function and stability of many oncogene products and cell‐signaling molecules. The Hsp90 inhibitor geldanamycin (GA) strongly binds to the N‐terminal ATP binding site of Hsp90,( 26 , 27 ) which prevents ATP binding and completion of client protein refolding, thereby disrupting its chaperone function( 28 , 29 , 30 ) for steroid hormone receptor,( 31 ) p53,( 32 ) oncogenic tyrosine kinases (including v‐src),( 33 ) serine/threonine kinases (including Raf‐1),( 34 ) and the signal transduction kinases ErbB‐2( 30 ) and serine/threonine kinase Akt. Akt regulates cell survival via phosphorylation of multiple substrates involved in the regulation of apoptosis. For example, it inhibits apoptosis by regulating the transcriptional activity of both nuclear factor‐κB( 35 , 36 ) and members of the Forkhead transcription factor family,( 37 ) and by phosphorylation (inhibition) of the pro‐apoptotic mediators caspase‐9( 38 ) and BAD (a Bcl‐2 homolog).( 39 , 40 ) That these molecules can be recovered from heterocomplexes containing Hsp90 suggests that destabilization of protein–Hsp90 multimolecular complexes causes several important signaling proteins to undergo rapid degradation via the ubiquitin‐dependent proteasomal mechanism.( 29 , 41 )
The expression of hsp90 is upregulated in tumors compared with normal tissue,( 42 , 43 ) and tumor cells are particularly sensitive to Hsp90 inhibition.( 44 ) Furthermore, the ability of a Hsp90 inhibitor to enhance the radiosensitivity of tumor cells( 45 , 46 , 47 ) and the anticancer effect of chemotherapy( 48 ) has been reported in a multicenter phase I clinical trial.( 49 , 50 , 51 , 52 ) But whereas the function of Hsp90 as a chaperone during heat stress and its role in mediating thermotolerance during hyperthermia therapy is generally recognized, the effect of Hsp90 inhibition on the cellular response to hyperthermia has not been fully investigated.
In the present study, we examined the effect of an Hsp90 inhibitor, GA, on the thermosensitivity of melanoma cells in vitro and in vivo. Our findings suggest that the combined use of self‐regulating, FMP‐mediated hyperthermia with Hsp90 inhibition is a potentially useful approach to the treatment of some cancers, including melanoma.
Materials and methods
Tumor cell line and reagent. Mouse B16 melanoma cells (Stratagene, La Jolla, CA, USA) were cultured in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin G, 100 µg/mL streptomycin, and 2 mg/mL amphotericin B) at 37°C under an atmosphere of 5% CO2, 95% air. Confluent cells were detached with Hank's balanced salt solution (pH 7.4) containing 0.05% trypsin and 5 mM ethylenediaminetetraacetic acid (Life Technologies, Grand Island, NY, USA) and subcultured.
Geldanamycin (Alexis Biochemicals, Lausen, Switzerland), a specific Hsp90 inhibitor, was dissolved in dimethyl sulfoxide (Kanto, Tokyo, Japan) to a concentration of 10 mM and stored in the dark at –20°C. For experimentation, the GA stock solution was diluted to the desired concentration in medium immediately before use.
Cell viability assay. Cell viability was evaluated using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA), which is a colorimetric assay for measuring numbers of viable cells. Briefly, B16 melanoma cells were cultured nearly to confluence in 96‐well, flat‐bottomed microplates (Cellstar, Greiner‐Bio‐One, Frickenhausen, Germany). The cells were then incubated in medium containing GA (0.2 µM) for 16 h at 37°C, after which they were heated at 43°C for 30 min. The cells were incubated in the presence of GA for an additional 8 h. The GA‐containing medium was then removed, the cells were washed with phosphate‐buffered saline (PBS; pH 7.4), and fresh drug‐free medium was added. Thereafter, CellTiter 96 Aqueous One Solution reagent was added to the wells, and the absorbance at 490 nm was measured 1 h later using a fluorescence microplate reader (Genios; Tecam, Salzburg, Austria). For each treatment group, the numbers of viable cells were normalized to the numbers of untreated control cells and reported as percentage viability. Each experiment was carried out five times.
TUNEL analysis. We used Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling (TUNEL) analysis to assess the effect of GA on hyperthermia‐induced apoptosis among melanoma cells. Nearly confluent cells were cultured for 16 h at 37°C in 24‐well, flat‐bottomed microplates containing plastic coverslip inserts (Celldesk LF; Sumitomo Bakelite Company, Tokyo, Japan) in medium with or without 0.2 µM GA. Thereafter, the cells were heated at 43°C for 30 min and incubated for an additional 1 h at 37°C. The medium was removed, and the cells were washed with PBS and fixed with 4% formalin in PBS. Apoptotic cells were detected using an Apop Tag Peroxidase In Situ Apoptosis Detection kit (S7100; Intergen Company, New York, NY, USA) in which 3‐amino‐9‐ethylcarbazole served as the colorant. After staining, 10 randomly selected high‐power (×400) fields were observed in each slide, and apoptotic cells were counted.
Western blotting. Expression of Akt or Hsp90 was evaluated by western blotting. Briefly, 1 h after carrying out the hyperthermia assay with or without 0.2 µM GA, the cultured B16 melanoma cells were washed three times with PBS and then lysed by incubation for 30 min on ice in 400 µL lysis buffer (20 mM Tris‐HCl pH 7.4, 0.15 M NaCl, 0.1% sodium dodecylsulfate, 1% sodium deoxycolate, 1% Triton‐X100, 5 mM ethylenediaminetetraacetic acid) containing a protease inhibitor cocktail tablet (Complete Mini; Roche, Mannheim, Germany). The resultant soluble cell extract was centrifuged for 10 min at 10 000 g , after which the supernatant was collected, boiled in Laemmli sample buffer (2% sodium dodecylsulfate, 10% glycerol, 100 mM dithiothreitol, 60 mM Tris‐HCl pH 6.8, 0.001% bromphenole blue), and subjected to 10% polyacrylamide gel electrophoresis. The separated proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon‐P; Millipore, Bedford, MA, USA), blocked for 1 h with 1% gelatin in Tris‐buffered saline (TBS) containing 0.2% Tween 20, and probed using rabbit anti‐Akt antibody (1:250 dilution; Rockland, Gilbertsville, PA, USA) or anti‐Hsp90 antibody (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After three washes with 0.2% Tween 20‐TBS, the blots were incubated for 1 h with horseradish peroxidase‐conjugated donkey anti‐rabbit IgG (1:10 000 dilution; Chemicon International, Temecula, CA, USA), again washed three times with 0.2% Tween 20‐TBS, visualized with enhanced chemiluminescence (ECL) (Amersham, Buckinghamshire, UK), and exposed to RX‐U X‐ray film (Fuji, Tokyo, Japan), which was developed using an SRX‐101 automatic processor (Konica, Tokyo, Japan). The 60‐kDa protein was considered to be Akt. Using National Institutes of Health image software, the results were normalized to the baseline optical densities obtained with untreated cells.
Animal model. Female 6‐week‐old C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan) or Charles River Japan (Yokohama, Japan). To prepare tumor‐bearing animals, cell suspensions consisting of approximately 1.0 × 106 melanoma cells in 100 µL PBS were injected subcutaneously into the backs of C57BL/6 mice. After approximately 10 days, the melanoma nodules had grown to approximately 5 mm in diameter and were used for experimentation. Tumor diameters were measured every 2 days, and their sizes were determined by applying the following formula:
| Tumor size = 0.5 × (length + width, in mm). |
Animal experiments were carried out according to the principles laid down in the ‘Guide for the Care and Use of Laboratory Animals’ prepared under the direction of the Office of the Prime Minister of Japan.
Injection of FMP and induction of tumor hyperthermia. We previously demonstrated our ability to induce hyperthermia magnetically using FMP with a Tc (43°C) low enough to mediate automatic temperature control.( 22 ) In the present study we used FMP (average diameter 100 µm; TDK Corporation, Tokyo, Japan) composed of Fe2O3, CuO, ZnO, and MgO (49:7:30:14 mol%, respectively) and created a magnetic field (600 A, 188 kHz) using a horizontal coil (diameter 10 cm, two turns) with an induction heating system (Hot Shot 5; Ameritherm, Scottsville, NY, USA).
After the melanoma nodules had grown to approximately 5 mm in diameter, mice were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg bodyweight), and approximately 500 mg FMP in 500 µL PBS was injected into the center of each nodule using a syringe with a 21‐gauge needle. The mice were then divided into four groups of 9–10 mice each: group I mice were left untreated (control group); group II mice were administered a single 30‐min hyperthermia treatment; group III mice were administered GA (1 mg/kg intraperitoneally in a final volume of 100 µL);( 53 ) and group IV mice were administered GA 4 h after a single hyperthermia treatment.
In every experiment, each mouse was anesthetized by intraperitoneal injection of pentobarbital and placed inside the coil such that the tumor was positioned at the center of the coil.
To determine whether hyperthermia induces apoptosis within tumors, on day 20 after injecting the FMP three mice from each of groups I to IV were killed, and the tumors were removed, fixed in 10% neutral buffered formalin for 18 h at 4°C, and embedded in paraffin. After preparing 3‐µm sections, the TUNEL method was used to identify apoptotic cells using an Apop Tag Peroxidase In Situ Apoptosis Detection kit, followed by nuclear counterstaining with hematoxylin. After staining, ten random fields (three mice in each group, total 30 fields in each group) under constant magnification (×400) were observed, and the percentage of apoptotic cells was evaluated.
Statistics. Group data are expressed as mean ± SD. The statistical significance of differences among groups was assessed using one‐way anova with Scheffe's multiple comparison tests. Differences in survival rates were analyzed using the log‐rank test in Stat View 5.0 (Macintosh, Barkeley, CA, USA).
Results
Inhibition of Hsp90 induces thermosensitization of melanoma cells. To assess GA‐induced modulation of the effect of hyperthermia on cultured melanoma cells, we used cell viability assays to examine cell viability. We found that in the absence of hyperthermia, cell viability in the presence GA was 83.5 ± 10.1% and hyperthermia alone was 79.3 ± 14.0%, which was significantly higher than the 50.9 ± 12.1% viability seen when cells were exposed to GA + hyperthermia (P < 0.05) (Fig. 1). GA thus increased hyperthermia‐induced cell death by a factor of 1.68 ± 0.23.
Figure 1.
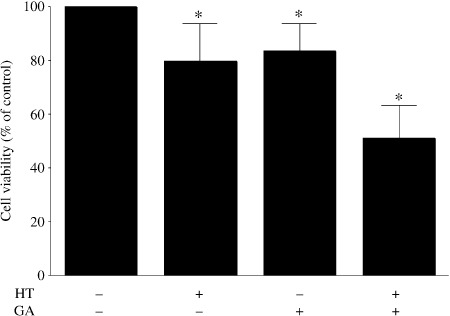
Geldanamycin (GA) sensitizes cultured melanoma cells to hyperthermia (HT). Cells were incubated with GA (0.2 µM) for 16 h at 37°C, exposed to 43°C or 37°C for 30 min, and then incubated for an additional 8 h at 37°C. The cell viabilities were normalized to that of untreated control cells. Symbols depict mean ± SD of five measurements. *P < 0.05 versus control.
Effect of inhibiting the Hsp90 signal transduction pathway. Expression of Akt, which is a client protein of Hsp90, was evaluated by western blotting using anti‐Akt antibody. Exposing melanoma cells to GA or hyperthermia alone led to a reduction in Akt levels, whereas the combination of GA + hyperthermia abolished expression of Akt protein completely (Fig. 2a). The expression of Hsp90 increased after hyperthermia with or without GA treatment. Although the level of Hsp90 with GA alone was slightly increased compared to the baseline, this might be a compensatory increase in response to inhibition by GA. Figure 2(b) shows the relative levels of Akt expression in cells treated with GA, hyperthermia, or GA + hyperthermia normalized to the basal Akt levels seen in untreated control cells. When administered together, GA and hyperthermia reduced expression of Akt by 81% compared to the control.
Figure 2.
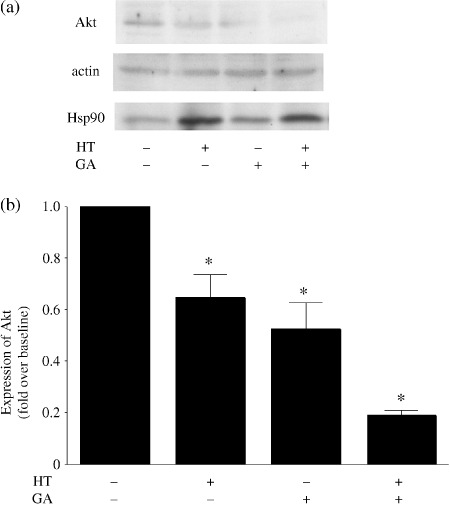
(a) Western blotting analysis of the individual and combined effects of hyperthermia (HT) and geldanamycin (GA) on Akt expression in cultured melanoma cells. (b) Akt levels were normalized to the control level obtained with untreated cells. Measurements were carried out five times in each. *P < 0.05 versus control.
Hyperthermia and GA induce apoptosis. To determine whether the increase in thermosensitivity induced by GA reflected an increased incidence of apoptosis, the TUNEL method was used to identify apoptotic cells among cultured melanoma cells. We found that the incidence of apoptosis was significantly increased when cells were treated with GA (44.0 ± 16.5 vs 76.2 ± 20.0 apoptotic cells per high‐powered field). Moreover, the incidence of apoptosis induced by GA + hyperthermia was significantly greater than that induced by hyperthermia alone, confirming that the effect of hyperthermia was enhanced by GA (Table 1).
Table 1.
Incidence of TUNEL positivity in the four experimental groups
| Group | TUNEL‐positive cells (apoptotic cells/high‐powered field) |
|---|---|
| Control | 10.3 ± 5.7 |
| Hyperthermia alone | 53.9 ± 9.6* |
| Geldanamycin alone | 44.0 ± 16.5* |
| Geldanamycin + hyperthermia | 76.2 ± 20.0** |
The data depict mean ± SD.
P < 0.05 versus control.
P < 0.05 versus hyperthermia alone.
Effect of GA in combination with FMP‐mediated hyperthermia in a mouse melanoma model. The schematic diagram in Figure 3 illustrates our experimental system. Tumor‐bearing mice were anesthetized by intraperitoneal injection of pentobarbital, after which each was placed such that the tumor was positioned at the center of the coil. When FMP previously injected into the tumor were exposed to the magnetic field, the temperature within the tumor increased sharply, reaching Tc within approximately 7 min, and was then maintained in the vicinity of Tc with little deviation during the entire period of exposure to the magnetic field (Fig. 4). Rectal temperature remained at around 37°C throughout the exposure period, although it did increase slightly. Thus, our hyperthermia system using FMP appears able to mediate selective, localized heating of the tumor without affecting healthy tissue.
Figure 3.
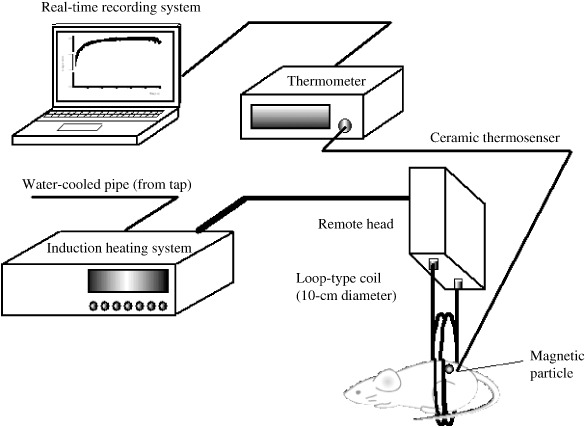
System for induction of ferromagnetic particle (FMP)‐mediated hyperthermia. FMP were injected into the tumor and exposed to a magnetic field (600 A, 188 kHz) created using a horizontal coil in an induction heating system. In some experiments tumor and rectal temperatures were measured continuously during exposure to the magnetic field using a ceramic thermocouple and recorded on a computer system.
Figure 4.
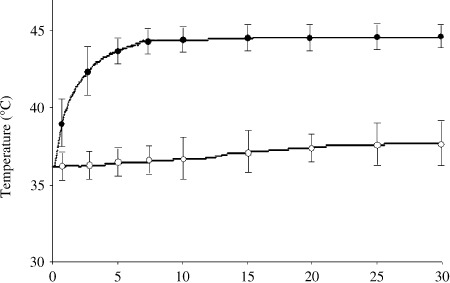
Hyperthermia induced using ferromagnetic particles (FMP) in vivo. FMP were injected directly into subcutaneous B16 melanoma tumors in mice, after which the mice were exposed to a magnetic field for 30 min. Tumor ( ) and rectal (
) and rectal ( ) temperatures were measured using a ceramic thermocouple. Symbols represent mean ± SD of five mice.
) temperatures were measured using a ceramic thermocouple. Symbols represent mean ± SD of five mice.
Figure 5 shows the time courses of tumor growth in the four treatment groups. No tumor regression was seen in groups I (control; no treatment), II (hyperthermia alone), or III (GA alone). By contrast, in group IV, which received GA + hyperthermia, tumor growth was significantly suppressed (P < 0.05). Indeed, complete regression of the tumor was observed in five of nine mice treated (56%).
Figure 5.
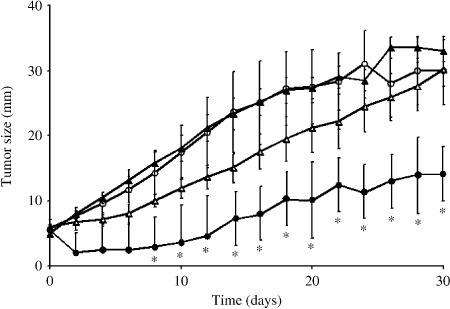
Time courses of tumor growth in groups I ( ; control, n = 10), II (
; control, n = 10), II (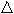 ; one hyperthermia treatment, n = 10), III (
; one hyperthermia treatment, n = 10), III ( ; geldanamycin treatment alone, n = 10), and IV (
; geldanamycin treatment alone, n = 10), and IV ( ; geldanamycin + hyperthermia, n = 9). Symbols depict mean ± SD. *P < 0.05 versus group I (control).
; geldanamycin + hyperthermia, n = 9). Symbols depict mean ± SD. *P < 0.05 versus group I (control).
To determine whether the effect of hyperthermia reflects an increased incidence of apoptosis, tumors were removed from another set of three mice in groups I–IV, and the TUNEL method was used to identify apoptotic melanoma cells (Fig. 6). We found that the incidence of apoptosis was significantly greater in tumors from group IV mice (109.6 ± 31.1%) than in those from group I mice (14.6 ± 4.4%) (Table 2). Immune cells, such as natural killer cells or lymphocytes, would infiltrate the area surrounding apoptotic cells; however, this is difficult to identify using only TUNEL staining with nuclear counterstaining. Apoptosis should have occurred shortly after the treatment; however, it was still observed well after the treatment, associated with some necrosis at the center of the tumors. We speculate that the initial treatment of hyperthermia and GA induced apoptosis, and this anticancer effect led to secondary factors. Hyperthermia is thought to decrease the vascular density in tumors, which indicates the suppression of nutritive blood vessel neogenesis in tumors, and direct damage of blood vessels may be involved in the induction of apoptosis and necrosis.( 54 ) We speculate that the initial treatment induced apoptosis, and then this affected ischemia resulting from destruction of the tumor vessels or inhibition of new vessel formation, thereby making the cells more vulnerable to apoptosis. Actually, a remnant tumor was examined in group IV (not complete regression [CR] mice because the tumors had disappeared), and the incidence of apoptosis was seen to be greater.
Figure 6.
The TUNEL method was used to identify apoptotic melanoma cells, which stained red in groups (a) I, (b) II, (c) III, and (d) IV 20 days after ferromagnetic particle injection. The incidence of apoptosis among the target tumors was significantly greater in (d) group IV than in (a) group I. Magnification, ×400.
Table 2.
Incidence of TUNEL positivity in the tumor in four experimental groups
| Group | TUNEL‐positive cells (apoptotic cells/high‐powered field) |
|---|---|
| Control | 14.6 ± 4.4 |
| Hyperthermia alone | 50.8 ± 12.4* |
| Geldanamycin alone | 32.4 ± 13.4* |
| Geldanamycin + hyperthermia | 109.6 ± 31.1** |
The data depict mean ± SD.
P < 0.05 versus control.
P < 0.05 versus hyperthermia alone.
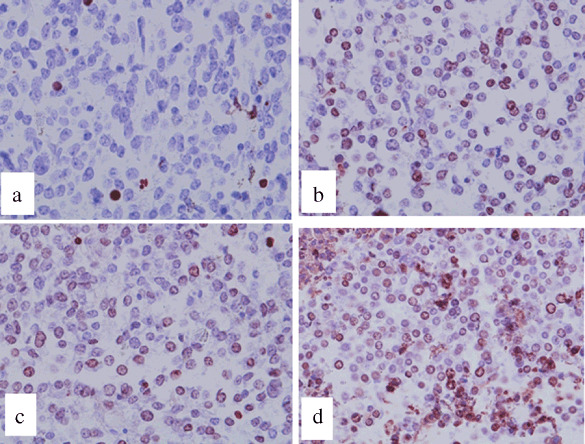
The survival rates among tumor‐bearing mice observed for a period of 40 days are shown in Figure 7. In groups I (control), II (hyperthermia alone), and III (GA alone), all of the mice died within approximately 30 days as a result of pulmonary metastases and enlargement of the tumor at the inoculation site. On the other hand, survival was significantly prolonged in group IV mice (GA + hyperthermia combination treatment) such that four mice remained alive at the end of the 40‐day observation period.
Figure 7.
Survival rates among tumor‐bearing mice observed for a period of 40 days after injection of ferromagnetic particles (FMP):  , group I (n = 10);
, group I (n = 10);  , group II (n = 10);
, group II (n = 10);  , group III (n = 10); and
, group III (n = 10); and  , group IV (n = 9). Survival in group IV was significantly prolonged compared to the other groups (P < 0.05 versus all other groups).
, group IV (n = 9). Survival in group IV was significantly prolonged compared to the other groups (P < 0.05 versus all other groups).
Discussion
Hyperthermia is an important part of cancer treatment; however, hyperthermia alone often fails to eliminate advanced‐stage tumors. This failure is likely a result of inherent thermotolerance mediated by Hsp family proteins. In fact it is generally recognized that Hsp90 induces thermotolerance through its chaperone activity during hyperthermia therapy. Nonetheless, the effect of inhibiting Hsp90 on thermotolerance and the susceptibility of tumor cells to hyperthermia has not been fully investigated.
We previously described a treatment system in which hyperthermia is magnetically induced using FMP. The system was designed to produce heat through production of eddy currents and to have a Tc of only 43°C, low enough to mediate automatic temperature control. We were further able to show that the hyperthermia induced with this system exerted an antitumor effect in a mouse melanoma model. This implies that FMP‐mediated hyperthermia selectively heated the tumor tissue, and that accurate control of tumor temperature could be achieved by setting an appropriate Tc. In the present study, we used GA to assess the effect of inhibiting Hsp90 on the thermosensitivity of melanoma cells and the feasibility using a novel combination therapy composed of FMP‐mediated, self‐regulating hyperthermia and Hsp90 inhibition for the treatment of malignant melanoma. We found that treating cultured melanoma cells with GA increased their thermosensitivity and reduced the expression of Akt. Moreover, when mice bearing melanomas were administered a single treatment with GA + hyperthermia, more than half (56%) showed complete regression of the tumor. This suggests that inhibiting Hsp90 enhances susceptibility to hyperthermia, thereby increasing its antitumor effect.
Geldanamycin is a novel antiproliferative agent that inhibits Hsp90 activity by binding to its N‐terminal ATP binding site.( 26 ) Hsp90 interacts with a variety of client proteins, many of which are involved in mitogenic and survival signaling, and plays a key role in the maintenance of their proper conformation.( 55 ) Simultaneous depletion of one or more of these client proteins likely contributes to the antitumor activity of Hsp90 inhibitors.( 56 ) The fact that Hsp90 inhibitors affect multiple signaling pathways also makes them attractive potential therapeutic agents for the treatment of malignant tumors. In that regard, it has been shown that an important feature of the initiation of apoptosis is downregulation of Akt, a critical mediator of cell survival known to associate with Hsp90.( 57 , 58 , 59 ) The mechanisms by which Akt promotes cell survival include phosphorylation, and thus inactivation, of pro‐apoptotic proteins such as BAD, caspase‐9, and members of the Forkhead family of transcription factors. In addition, Akt appears to be involved in the activation of pro‐survival signaling such as that in the nuclear factor‐κB pathway. Akt, which is a client protein of Hsp90, can be recovered from heterocomplexes containing Hsp90, suggesting that destabilization of protein–Hsp90 multimolecular complexes by treatment with GA causes these client proteins to undergo rapid degradation via the ubiquitin‐dependent proteasomal mechanism,( 29 , 41 ) resulting in decreased Akt levels in cells. In the present study, we examined the effect of an Hsp90 inhibitor (GA) on the cellular levels of Akt during hyperthermia and found that the GA‐induced decline in Akt levels was exacerbated by hyperthermia and correlated with an increased incidence of apoptosis. Thus, Hsp90 inhibition appears to enhance the effect of hyperthermia on the incidence of apoptosis. In general, heat shock results in a rapid but transient increase in Akt phosphorylation; however, Akt expression did not increase with hyperthermia alone in the present study. We speculate that the transiently increased phosphorylation of Akt might not be due to an increase in overall protein production at this time point. Furthermore, according to the cell proliferation assay (Fig. 1), cell viability was decreased 8 h after hyperthermia. This means that cell death, including apoptosis, has already occurred by 8 h after hyperthermia. Cell death may occur through a variety of mechanisms; it is possible that hyperthermia might affect the upstream Akt signal‐transduction pathway, resulting in slight downregulation of Akt. Meanwhile, the cellular level of Akt almost disappeared (Fig. 2b) with GA + hyperthermia treatment, although cell viability remained at approximately 50% of the control (Fig. 1). This might be influenced by the protective effect of hyperthermia increasing the expression of other Hsp (e.g. Hsp72), which may render the tumor cells hyperthermia resistant.
Using tumor‐bearing mice, we observed that the combined use of the Hsp90 inhibitor GA with our hyperthermia system( 22 ) exerted a significant antitumor effect. But although tumor growth was suppressed by treatment with GA + hyperthermia, the hepatic toxicity of GA may have diminished the beneficial effect,( 60 ) although we did not confirm this histologically. Data from ongoing phase I trials have already indicated that several GA derivatives, including 17‐allylamino‐17‐demethoxygeldanamycin, are well tolerated in adults with refractory solid tumors at doses that modulate Hsp90 client protein levels in lymphocytes.( 51 ) Our findings thus provide preclinical support for a novel approach to anticancer treatment involving the use of a Hsp90 inhibitor in combination with hyperthermia. It would be of great interest to know whether similar beneficial effects could be obtained with 17‐allylamino‐17‐demethoxygeldanamycin in combination with hyperthermia in our animal model.
An intriguing aspect of using a Hsp90 inhibitor as a thermosensitizing agent is the different effects in normal and tumor cells. In a phase I clinical trial, Hsp90 inhibition was well tolerated in patients, even though Hsp90 is also present in normal cells. This might be because Hsp90 inhibitors bind to Hsp90 with 100‐times greater affinity in tumor cells, where Hsp90 is in an activated state as a part of the multichaperone complex.( 43 ) Furthermore, Hsp90α, but not the β isoform, is expressed extracellularly and binds to matrix metalloproteinase 2, thereby activating its enzyme activity and enhancing the invasiveness of tumor cells.( 61 ) Inhibition of extracellular Hsp90α reduces both matrix metalloproteinase 2 activity and invasiveness, suggesting that the therapeutic effect of Hsp90 inhibition may reflect both a reduction in the invasiveness of tumor cells and an increase in thermosensitivity.
In summary, our findings suggest that Hsp90 inhibition sensitizes cancer cells to hyperthermia and that a novel combination of FMP‐mediated local hyperthermia and Hsp90 inhibition is a potentially effective approach to anticancer treatment.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Scientific Research (B) from the Japan Society for the Promotion of Science 18390373. The authors thank Ms Mitsuko Sato and Jun Kodama for their secretarial support, and Mr Masaaki Chihara and Hitoshi Iwaya for useful discussion.
References
- 1. Cavaliere R, Ciocatto EC, Giovanella BC et al . Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967; 20: 1351–81. [DOI] [PubMed] [Google Scholar]
- 2. Overgaard K, Overgaard J. Investigations on the possibility of a thermic tumour therapy. I. Short‐wave treatment of a transplanted isologous mouse mammary carcinoma. Eur J Cancer 1972; 8: 65–78. [DOI] [PubMed] [Google Scholar]
- 3. Lefor AT, Makohon S, Ackerman NB. The effects of hyperthermia on vascular permeability in experimental liver metastasis. J Surg Oncol 1985; 28: 297–300. [DOI] [PubMed] [Google Scholar]
- 4. Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep‐seated tumors. I. Studies on thermometry. Cancer 1987; 60: 121–7. [DOI] [PubMed] [Google Scholar]
- 5. Van Der Zee J. Heating the patient: a promising approach? Ann Oncol 2002; 13: 1173–84. [DOI] [PubMed] [Google Scholar]
- 6. Abe M, Hiraoka M, Takahashi M et al . Multi‐institutional studies on hyperthermia using an 8‐MHz radiofrequency capacitive heating device (Thermotron RF‐8) in combination with radiation for cancer therapy. Cancer 1986; 58: 1589–95. [DOI] [PubMed] [Google Scholar]
- 7. Hiraoka M, Nishimura Y, Nagata Y et al . Site‐specific phase I, II trials of hyperthermia at Kyoto University. Int J Hyperthermia 1994; 10: 403–10. [DOI] [PubMed] [Google Scholar]
- 8. Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993; 9: 51–68. [DOI] [PubMed] [Google Scholar]
- 9. Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn J Cancer Res 1996; 87: 1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. Jpn J Cancer Res 1998; 89: 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minamimura T, Sato H, Kasaoka S et al . Tumor regression by inductive hyperthermia combined with hepatic embolization using dextran magnetite‐incorporated microspheres in rats. Int J Oncol 2000; 16: 1153–8. [DOI] [PubMed] [Google Scholar]
- 12. Jordan A, Scholz R, Maier‐Hauff K et al . Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J Magnetism Magn Mater 2001; 225: 118–26. [Google Scholar]
- 13. Ito A, Tanaka K, Kondo K et al . Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003; 94: 308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanaka K, Ito A, Kobayashi T et al . Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer 2005; 116: 624–33. [DOI] [PubMed] [Google Scholar]
- 15. Johannsen M, Gneveckow U, Eckelt L et al . Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia 2005; 21: 637–47. [DOI] [PubMed] [Google Scholar]
- 16. Wust P, Gneveckow U, Johannsen M et al . Magnetic nanoparticles for interstitial thermotherapy: feasibility, tolerance and achieved temperatures. Int J Hyperthermia 2006; 22: 673–85. [DOI] [PubMed] [Google Scholar]
- 17. Jordan A, Scholz R, Maier‐Hauff K et al . The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol 2006; 78: 7–14. [DOI] [PubMed] [Google Scholar]
- 18. Lilly MB, Brezovich IA, Atkinson WJ. Hyperthermia induction with thermally self‐regulated ferromagnetic implants. Radiology 1985; 154: 243–4. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Kida Y, Tanaka T, Kageyama N, Kobayashi H, Amemiya Y. Magnetic induction hyperthermia for brain tumor using ferromagnetic implant with low Curie temperature. I. Experimental study. J Neurooncol 1986; 4: 175–81. [DOI] [PubMed] [Google Scholar]
- 20. Müller‐Schulte D. Means for the selective tumor therapy as well as processes for the synthesis and their application. German Patent DE3502998A1. 1986.
- 21. Doerschuk CM, Allard MF, Hogg JC. Neutrophil kinetics in rabbits during infusion of zymosan‐activated plasma. J Appl Physiol 1989; 67: 88–95. [DOI] [PubMed] [Google Scholar]
- 22. Saito H, Mitobe K, Ito A et al . Self‐regulating hyperthermia induced using thermosensitive ferromagnetic material with a low Curie temperature. Cancer Sci 2008; 99: 805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman BC, Morimoto RI. The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj‐1 have distinct roles in recognition of a non‐native protein and protein refolding. EMBO J 1996; 15: 2969–79. [PMC free article] [PubMed] [Google Scholar]
- 24. Schneider C, Sepp‐Lorenzino L, Nimmesgern E et al . Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A 1996; 93: 14 536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schumacher RJ, Hansen WJ, Freeman BC, Alnemri E, Litwack G, Toft DO. Cooperative action of Hsp70, Hsp90, and DnaJ proteins in protein renaturation. Biochemistry 1996; 35: 14 889–98. [DOI] [PubMed] [Google Scholar]
- 26. Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP‐binding site in the Hsp90 molecular chaperone. Cell 1997; 90: 65–75. [DOI] [PubMed] [Google Scholar]
- 27. Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 1997; 89: 239–50. [DOI] [PubMed] [Google Scholar]
- 28. Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c‐erbB‐2 receptor protein‐tyrosine kinase induced by geldanamycin. J Biol Chem 1996; 271: 22 796–801. [DOI] [PubMed] [Google Scholar]
- 29. Stancato LF, Silverstein AM, Owens‐Grillo JK, Chow YH, Jove R, Pratt WB. The hsp90‐binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem 1997; 272: 4013–20. [DOI] [PubMed] [Google Scholar]
- 30. Xu W, Mimnaugh E, Rosser MF et al . Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem 2001; 276: 3702–8. [DOI] [PubMed] [Google Scholar]
- 31. Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 1997; 18: 306–60. [DOI] [PubMed] [Google Scholar]
- 32. Blagosklonny MV, Schulte TW, Nguyen P, Mimnaugh EG, Trepel J, Neckers L. Taxol induction of p21WAF1 and p53 requires c‐raf‐1. Cancer Res 1995; 55: 4623–6. [PubMed] [Google Scholar]
- 33. Xu Y, Lindquist S. Heat‐shock protein hsp90 governs the activity of pp60v‐src kinase. Proc Natl Acad Sci USA 1993; 90: 7074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell‐free system. J Biol Chem 1993; 268: 21 711–16. [PubMed] [Google Scholar]
- 35. Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF‐κB activation by tumour necrosis factor requires the Akt serine‐threonine kinase. Nature 1999; 401: 82–5. [DOI] [PubMed] [Google Scholar]
- 36. Romashkova JA, Makarov SS. NF‐κB is a target of AKT in anti‐apoptotic PDGF signalling. Nature 1999; 401: 86–90. [DOI] [PubMed] [Google Scholar]
- 37. Brunet A, Bonni A, Zigmond MJ et al . Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96: 857–68. [DOI] [PubMed] [Google Scholar]
- 38. Cardone MH, Roy N, Stennicke HR et al . Regulation of cell death protease caspase‐9 by phosphorylation. Science 1998; 282: 1318–21. [DOI] [PubMed] [Google Scholar]
- 39. Datta SR, Dudek H, Tao X et al . Akt phosphorylation of BAD couples survival signals to the cell‐intrinsic death machinery. Cell 1997; 91: 231–41. [DOI] [PubMed] [Google Scholar]
- 40. Del Peso L, González‐García M, Page C, Herrera R, Nuñez G. Interleukin‐3‐induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278: 687–9. [DOI] [PubMed] [Google Scholar]
- 41. Pratt WB. The hsp90‐based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 1998; 217: 420–34. [DOI] [PubMed] [Google Scholar]
- 42. Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat‐shock proteins in human tumor cells. Int J Cancer 1992; 51: 613–19. [DOI] [PubMed] [Google Scholar]
- 43. Kamal A, Thao L, Sensintaffar J et al . A high‐affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003; 425: 407–10. [DOI] [PubMed] [Google Scholar]
- 44. Bisht KS, Bradbury CM, Mattson D et al . Geldanamycin and 17‐allylamino‐17‐demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90‐mediated intracellular signaling and cytotoxicity. Cancer Res 2003; 63: 8984–95. [PubMed] [Google Scholar]
- 45. Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. Int J Radiat Biol 2003; 79: 973–80. [DOI] [PubMed] [Google Scholar]
- 46. Machida H, Nakajima S, Shikano N et al . Heat shock protein 90 inhibitor 17‐allylamino‐17‐demethoxygeldanamycin potentiates the radiation response of tumor cells grown as monolayer cultures and spheroids by inducing apoptosis. Cancer Sci 2005; 96: 911–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsumoto Y, Machida H, Kubota N. Preferential sensitization of tumor cells to radiation by heat shock protein 90 inhibitor geldanamycin. J Radiat Res 2005; 46: 215–21. [DOI] [PubMed] [Google Scholar]
- 48. Münster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy‐induced apoptosis in an RB‐ and schedule‐dependent manner. Clin Cancer Res 2001; 7: 2228–36. [PubMed] [Google Scholar]
- 49. Adams J, Elliott PJ. New agents in cancer clinical trials. Oncogene 2000; 19: 6687–92. [DOI] [PubMed] [Google Scholar]
- 50. Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17‐allylamino, 17‐demethoxygeldanamycin. Curr Cancer Drug Targets 2003; 3: 377–83. [DOI] [PubMed] [Google Scholar]
- 51. Banerji U, O'Donnell A, Scurr M et al . Phase I pharmacokinetic and pharmacodynamic study of 17‐allylamino, 17‐demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 2005; 23: 4152–61. [DOI] [PubMed] [Google Scholar]
- 52. Grem JL, Morrison G, Guo XD et al . Phase I and pharmacologic study of 17‐(allylamino)‐17‐demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol 2005; 23: 1885–93. [DOI] [PubMed] [Google Scholar]
- 53. Suganuma T, Irie K, Fujii E, Yoshioka T, Muraki T. Effect of heat stress on lipopolysaccharide‐induced vascular permeability change in mice. J Pharmacol Exp Ther 2002; 303: 656–63. [DOI] [PubMed] [Google Scholar]
- 54. Fajardo LF, Prionas SD. Endothelial cells and hyperthermia. Int J Hyperthermia 1994; 10: 347–53. [DOI] [PubMed] [Google Scholar]
- 55. Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti‐cancer agent: its molecular target and biochemical activity. Invest New Drugs 1999; 17: 361–73. [DOI] [PubMed] [Google Scholar]
- 56. Clarke PA, Hostein I, Banerji U et al . Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17‐allylamino‐17‐demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene 2000; 19: 4125–33. [DOI] [PubMed] [Google Scholar]
- 57. Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non‐small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 2001; 61: 3986–97. [PubMed] [Google Scholar]
- 58. Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf‐1–Hsp90 molecular complex results in destabilization of Raf‐1 and loss of Raf‐1‐Ras association. J Biol Chem 1995; 270: 24 585–8. [DOI] [PubMed] [Google Scholar]
- 59. Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 2000; 97: 10 832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amin K, Ip C, Jimenez L, Tyson C, Behrsing H. In vitro detection of differential and cell‐specific hepatobiliary toxicity induced by geldanamycin and 17‐allylaminogeldanamycin using dog liver slices. Toxicol Sci 2005; 87: 442–50. [DOI] [PubMed] [Google Scholar]
- 61. Eustace BK, Sakurai T, Stewart JK et al . Functional proteomic screens reveal an essential extracellular role for hsp90α in cancer cell invasiveness. Nat Cell Biol 2004; 6: 507–14. [DOI] [PubMed] [Google Scholar]


