Figure 5.
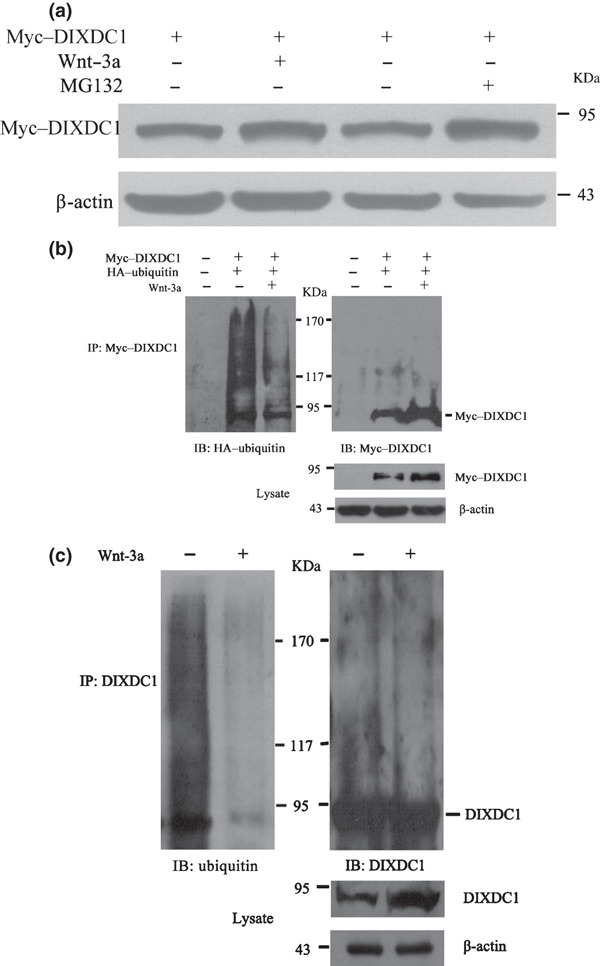
Wnt‐3a increased the DIX Domain Containing 1 (DIXDC1) protein level via inhibiting its ubiquitin‐mediated degradation. (a) steady‐state level of the myc‐tagged DIXDC1 protein was increased after carbobenzoxy‐l‐leucyl‐l‐leucyl‐l‐leucinal (MG132) (10μM) or Wnt‐3a (80 ng/mL) treatment. HeLa cells transiently transfected with the myc‐tagged DIXDC1 plasmid were treated with Wnt‐3a or MG132 and then subjected to immunoblotting analysis of DIXDC1 protein expression. Protein levels were normalized to β‐actin. (b) Wnt‐3a decreased the ubiquitination of the ectopically expressed DIXDC1 protein. HeLa cells were cotransfected with myc‐tagged DIXDC1 and hemagglutinin‐tagged ubiquitin constructs. Cells were treated with Wnt‐3a or phosphate‐buffered saline at 24 h after transfection. Cell extracts were subjected to immunoprecipitation using the anti‐myc antibody. Ubiquitinated myc–DIXDC1 was analyzed by immunoblotting using the anti‐HA antibody. Same blot was stripped and reprobed with the anti‐myc antibody. Steady‐state level of the myc–DIXDC1 protein was examined by western blotting using the anti‐myc antibody and normalized to β‐actin. (c) Wnt‐3a decreased the ubiquitination of endogenous DIXDC1. HeLa cell lysates were subjected to immunoprecipitation using the anti‐DIXDC1 rabbit polyclonal antibody. Ubiquitinated DIXDC1 was detected by immunoblotting using the anti‐ubiquitin antibody. After stripping, the same blot was stained with the anti‐DIXDC1 antibody. Endogenous DIXDC1 protein level was detected by western blot using the anti‐DIXDC1 antibody and normalized to β‐actin. Similar results were obtained in three independent experiments.
