Figure 1.
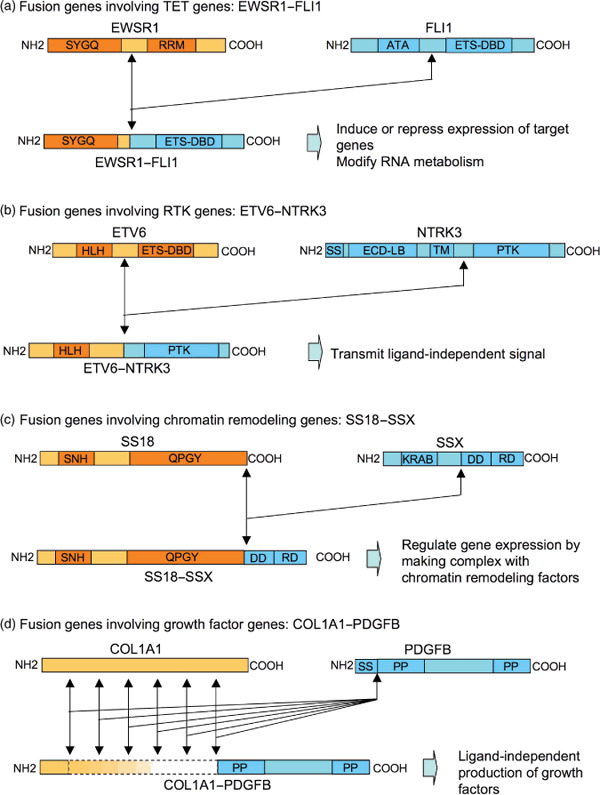
Structure and putative function of fusion proteins found in sarcomas. Representative cases are shown for each of four different types of fusion proteins. (a) Fusion genes involving TET genes: EWSR1–FLI1. The N‐terminus of EWSR1 containing a Ser‐Tyr‐Gly‐Gln rich region (SYGQ) fused with the C‐terminus of FLI1 containing Ets DNA‐binding domain (ETS–DBD). RRM, RNA recognition motif; ATA, amino‐terminal transactivation domain. (b) Fusion genes involving RTK genes: ETV6–NTRK3. The N‐terminus of ETV6 containing a basic helix‐loop‐helix domain (HLH) fused with the C‐terminus of NTRK3 containing a protein tyrosine kinase domain (PTK). SS, signal peptide; ECD‐LB, extracellular ligand‐binding domain; TM, transmembrane domain. (c) Fusion genes involving chromatin remodeling genes: SS18–SSX. The N‐terminus of SS18 containing an SS18 N‐terminal homology domain (SNH) and a Gln‐Pro‐Gly‐Tyr‐rich domain (QPGY) fused with the C‐terminus of SSX containing a dubbed divergent domain (DD) and an SSX repression domain (SSXRD). The former is highly divergent among the SSX protein family, while the latter has the highest similarity within the family. KRAB, Krüppel‐associated box‐like domain. (d) Fusion genes involving growth factor genes: COL1A1–PDGFB. The length of the N‐terminus of COL1A1 in the fusion protein is highly divergent, while the structure of PDGFB in the fusion protein is constant, containing an N‐terminal and C‐terminal propeptide (PP). SS, signal peptide.
