Abstract
Downregulation of the cyclin‐dependent kinase inhibitory protein p27 is frequently observed in various cancers due to enhancement of its degradation. We recently reported that p53‐inducible protein with RING‐H2 domain (Pirh2) is a novel ubiquitin ligase for p27, required for the ubiquitylation and consequent degradation of p27 protein. However, there is no reports about the involvement of Pirh2 in both p27 downregulation and pathogenesis in human cancers. In the present study, we investigated them using cultured cell lines and surgical specimens derived from human head and neck squamous cell carcinoma (HNSCC). Depletion of Pirh2 by short interfering RNA induced accumulation of p27 and inhibited the growth of cultured HNSCC cells. By immunohistochemical analysis in 57 cases of HNSCC specimens, higher levels of Pirh2 expression (labeling index ≥ 60%) were found in 61.4% of HNSCC in comparison with 0% of normal mucosa. In addition, 83.3% of HNSCC with lower p27 expression (labeling index < 20%) displayed high Pirh2 levels. Therefore, Pirh2 expression was inversely correlated with p27 expression. Finally, Pirh2 expression was well correlated with poor prognosis. These findings suggest that Pirh2 overexpression may have an important role in the development and maintenance of HNSCC at least partially through p27 degradation, and that Pirh2 may be a potential molecular target for human HNSCC. (Cancer Sci 2009; 100: 866–872)
Cyclin‐dependent kinases (CDK) and their inhibitor proteins play a critical role in cell cycle regulation. In G1 phase, the Cip/Kip‐type cyclin‐dependent kinase inhibitor p27Kip1 negatively regulates the cyclin D–CDK4 complex, which is thereafter activated to phosphorylate its target proteins such as retinoblastoma protein. Many studies have shown that a low expression level of p27 is found and associated with poor prognosis in a variety of human cancers, such as colon, breast, lung, stomach, and head and neck cancer.( 1 , 2 , 3 , 4 , 5 )
Deregulation of the retinoblastoma tumor‐suppressive pathway due to abnormal activation of cyclin‐CDK complex in G1‐S phase, which is caused by p27 downregulation, is suggested to promote cancer cell growth. The p27 protein is degraded via the ubiquitin proteasome system at late G1 phase. So far, S‐phase kinase‐associated protein (Skp) 2, an F‐box substrate recognition subunit of the Skp1‐Cull‐F‐box protein (SCF) ubiquitin ligase complex, and Kip1 ubiquitylation‐promoting complex (KPC), a cytoplasmic RING finger‐type E3 complex, were identified as critical E3 ligases for p27. The SCF–Skp2 complex recognizes Thr187‐phosphorylated p27 in collaboration with cdc kinase subunit (Cks) 1 to promote ubiquitylation of p27. Indeed, some studies have shown high expression levels of Skp2 and Cks1 proteins in non‐small cell lung carcinomas and oral squamous cell carcinomas.( 6 , 7 ) Because it was shown in these studies that a high expression level of Skp2 is correlated with a low expression level of p27, Skp2 and Cks1 are thought to be involved in the degradation of p27 in human cancers, whereas there has been no report about the alteration of KPC in human cancers. Recently, we identified p53‐inducible protein with RING‐H2 domain (Pirh2) as a novel E3 ligase for p27 using yeast two‐hybrid screening.( 8 ) Although Pirh2 was originally identified as a p53‐inducible and ‐interacting protein that promotes ubiquitylation and degradation of p53,( 9 ) we demonstrated that Pirh2 directly binds and ubiquitylates p27 in vivo as well as in vitro. Depletion of endogenous Pirh2 by short interfering RNA (siRNA) increased the steady‐state level of p27 and decelerated p27 turnover in COLO320DM (colon cancer) and T98G (glioblastoma) cells. Pirh2 expression was induced from late G1 to S phase whereas p27 was decreased in synchronization with Pirh2 accumulation. Furthermore, reduction of Pirh2 resulted in impairment of p27 degradation and inhibition of cell cycle progression at the G1–S transition in a p53‐independent manner. Because enhanced p27 downregulation is associated with poor prognosis in various human cancers, herein we examined Pirh2 expression and the role of p27 regulation in both cultured cells and surgical specimens of head and neck squamous cell carcinoma (HNSCC). Furthermore, we analyzed the relationships between Pirh2 expression and clinicopathological profiles and evaluated the involvement of Pirh2 in the prognosis of HNSCC patients.
Materials and Methods
Cases and classification. For this study, 57 cases of HNSCC, including adjacent normal epithelium, were retrieved from the Surgical Pathology Registry of Oral and Maxillofacial surgery, Hamamatsu University School of Medicine, Hamamatsu, Japan, from 1991 to 2002 (Supporting Table S1). Clinical information, including age, sex, primary sites, and outcome, were obtained from clinical records that are summarized in Supporting Table S1. Histopathological diagnosis was made and histological differentiation was evaluated according to the World Health Organization international histological classification of tumors.( 10 ) Cases were also classified according to the criteria of the Unio Internationalist Contra Cancrum [International Union Against Cancer] Tumor/Node/Metastasis (UICC TNM) classification for stage grouping.( 11 ) All of the patients had received curative surgical treatment, and none of the patients received any other treatment prior to surgery. The current study was done under the protocol approved by the Institutional Human Tissue Utilization Committee of Hamamatsu University School of Medicine.
Cell culture, reagents, and antibodies. HNSCC cell lines provided by the Japanese Cancer Research Resources Bank were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat‐inactivated fetal bovine serum. Primary antibodies used in this study were commercially available as follows: the monoclonal antibody against p27 (clone 57) was purchased from BD Biosciences (San Jose, CA, USA), the polyclonal antibody against human Pirh2 was from Calbiochem (San Diego, CA, USA), and the monoclonal antibody against β‐actin (clone AC‐15) was from Sigma (St Louis, MO, USA). A Pirh2 blocking peptide (Bethyl, Montgomery, TX, USA) was used to confirm the specificity of the anti‐Pirh2 antibody in the immunohistochemical study.
RNA interference. Human Pirh2 siRNA (5′‐CAUGCCCAACAG ACUUGUG‐3′) with 3′ dTdT overhangs were synthesized by Greiner Bio‐One (Frickenhausen, Germany). The control siRNA, used as a non‐silencing control, with no significant homology to rat, mouse, or human gene sequences (5′‐UUCUCCGAACGU GUCACGUTT‐3′), was purchased from JBioS (Saitama, Japan). The cells were transfected with siRNA oligonucleotides using RNAiMAX (Invitrogen, Paisley, UK) according to the manufacturer's protocol.
Western blot analysis. We examined the expression of Pirh2 and p27 in five cell lines of oral squamous cell carcinoma and in those transfected with Pirh2 siRNA by western blot analysis. Western blotting was carried out as described previously.( 8 ) A total of 30 µg of protein was subjected to 11% polyacrylamide gel electrophoresis followed by electroblotting onto PVDF membrane immobilon‐P (Millipore, Bedford, MA, USA). The primary antibodies were diluted as follows: Pirh2, 1:1000; p27, 1:2000; and β‐actin, 1:10000. Proteins were visualized using an enhanced chemiluminescence system (PerkinElmer Life Sciences, Wellesley, MA, USA).
Cell proliferation assay. At 24 h after control or Pirh2 siRNA transfection, 3.0 × 103 cells were plated onto a 96‐well multiwell plate and harvested at 12, 24, 36, 48, 60, and 72 h after plating. The number of cells was counted using a cell proliferation assay kit (Invitrogen) and fluorescence microplate reader. Doubling time was then calculated.
Cell cycle analysis. Cells were transfected with control or Pirh2 siRNA. Twenty‐four hours after transfection, cells were harvested using trypsin, rinsed, and fixed with 70% ethanol overnight. The cells were then rinsed with phosphate‐buffered saline and incubated with 0.5 mg/mL RNase A in phosphate‐buffered saline at 37°C for 20 min, and propidium iodide was added to a final concentration of 50 µg/mL. Stained cells were analyzed on a FACS array using FlowJo 6.4 software (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell block construction. To make the cell block preparations, HNSCC culture cells were harvested and fixed in 15% formaldehyde solution overnight. After the cell suspensions were centrifuged, the resultant pellets were dehydrated and embedded in paraffin. Serial sections of 3‐µm thickness were made and used for immunocytochemical analysis.
Immunohistochemistry. Immunohistochemical staining was carried out using Catalyzed Signal Amplification System 2 kits (Dako Japan, Kyoto, Japan). Sections were dewaxed with xylene, hydrated through graded ethyl‐alcohols, and rehydrated in water. Sections were pretreated in a microwave in citrate buffer (pH 6.0) for 15 min (only for p27 staining). An antibody against the Pirh2 protein was applied at a dilution of 1:4000, incubated at 4°C overnight, and anti‐p27 antibody was applied at a dilution of 1:1000, at room temperature for 30 min. Successive procedures, including application of the secondary antibody, amplification reagent, fluorescin isothiocyanate (FITC) peroxidase reagents, and colorization with diaminobenzidine solution, which were all contained in the Catalyzed Signal Amplification System 2 kit, were carried out according to the manufacturer's instructions. Sections were counterstained with hematoxylin.
For the evaluation of Pirh2 and p27 expression, the number of stained cells was counted in at least two fields among 1000 cells under a blind test (without knowledge of the clinical parameters and outcomes). The percentage of positively stained cells was designated as the labeling index (LI). For statistical analysis, the LI was scored and all cases were divided into the following groups based on the mean value of LI and the criteria described in previous reports: low Pirh2, <60% tumor cells with positive staining for Pirh2; high Pirh2, 60% or more tumor cells with positive staining for Pirh2; low p27, <20% tumor cells with positive staining for p27; and high p27, 20% or more for tumor cells with positive staining for p27.( 7 , 12 )
Statistical analysis. Statistical data analyses were carried out using the Statcel2 software package (OMS Publishing Inc., Saitama, Japan). A possible correlation between variables of the analyzed tumor samples was tested for association by Fisher's exact test and Spearman's rank correlation coefficient test. Patients’ survival data were used to determine a possible correlation between Pirh2 and p27 expression levels and survival time. Curves for survival were drawn according to the Kaplan–Meier method, and differences between the survival rates of two or three groups with different Pirh2 and p27 expression levels, respectively, were examined. The statistical significance of the data was measured using the Log‐rank test. Cox's proportional hazards regression analysis, which included tumor size, lymph node metastasis, differentiation, and Pirh2 status, was used to identify the multivariate predictive value of the prognostic factors. Univariate analysis was used to assess the relationship between survival time and potential categories of prognostic factors. P < 0.05 was required for significance.
Results
Involvement of Pirh2 in p27 expression and cell growth in HNSCC cell lines. Expression of endogenous Pirh2 and p27 protein was observed in HNSCC cell lines including Ho‐1‐u‐1, Ho‐1‐N‐1, HSC2, HSC3, and Ca9‐22 (Supporting Fig. S1). Among the five HNSCC cell lines, Ho‐1‐u‐1 cells showed the highest Pirh2 expression and Ho‐1‐N‐1 cells showed the lowest Pirh2 expression. Therefore, we used these two cell lines for subsequent experiments as representative. First, we investigated the effects of Pirh2 depletion on the level of p27. As shown in Figure 1(a), Pirh2 siRNA suppressed Pirh2 expression and accumulated p27 in HNSCC cell lines. Moreover, depletion of Pirh2 significantly inhibited cell growth and the G1 to S transition (Fig. 1b,c; Supporting Fig. S2). These results suggest that depletion of Pirh2 suppressed the degradation of p27 and thereby inhibited cell cycle progression in these cells.
Figure 1.
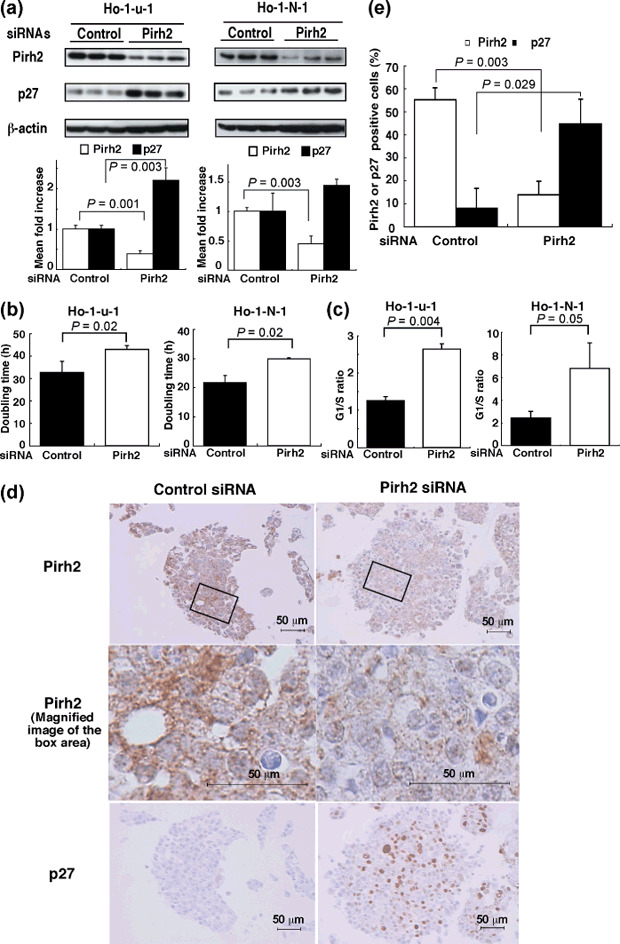
Depletion of Pirh2 increased p27 expression in head and neck squamous cell carcinoma (HNSCC) cell lines. (a) Depletion of Pirh2 induced p27 accumulation in HNSCC cells. Ho‐u‐1 and Ho‐1‐N‐1 cell lines were transfected with Pirh2 or control short interfering RNA (siRNA). Thirty µg of protein was subjected to western blot analysis using anti‐Pirh2 or anti‐p27 antibodies. β‐Actin blot was used as a loading control. The lower graph shows the quantitative density of Pirh2 and p27 protein expression detected by western blotting. (b) The effect of Pirh2 siRNA transfection on cell proliferation in vitro. Correlation was analyzed by Student's t‐test. Results are from three independent experiments. (c) Effects of Pirh2 depletion on cell cycle progression. P‐value of G1 to S ratios were analyzed by Student's t‐test. Results are from three independent experiments. (d) Accumulation of p27 by depletion of Pirh2 was detected by immunohistochemical analysis of Pirh2 and p27 in Ho‐1‐u‐1 cells. Ho‐1‐u‐1 cells were transfected with Pirh2 or control siRNA. Residual cells were packed as a cell block and then subjected to immunocytochemical analysis using anti‐Pirh2 or anti‐p27 antibodies (×200; magnified image, ×800). (e) Pirh2‐ or p27‐positive cells were counted and their rates calculated. Correlation was analyzed by Student's t‐test. The results are from three specimens.
Immunocytochemical analyses. Cell block sections of Ho‐1‐u‐1 cells that had been transfected with Pirh2 siRNA or control siRNA were subjected to immunocytochemistry using the Pirh2 and p27 antibodies. Positive staining for Pirh2 was localized predominantly to the cytoplasm and that for p27 was in the nucleus (Fig. 1d). As shown in Figure 1(d,e), the number and intensity of Pirh2‐stained cells in the Pirh2 knockdown cells were significantly decreased compared with the control cells (P = 0.003). Moreover, p27‐positive cells were significantly increased in the Pirh2 knockdown cells (P = 0.029) (Fig. 1d,e). Because these results were consistent with the data from western blotting analysis (Fig. 1a), it strongly suggested that this immunocytochemical analysis could be used to evaluate Pirh2 expression.
Enhanced expression of Pirh2 in HNSCC by immunohistochemistry. In the immunohistochemical analysis of representative human HNSCC tissues, Pirh2 protein was clearly stained in the tumor nests and localized to the cytoplasm (Fig. 2a, left, middle). However, when the Pirh2 antibody was incubated with Pirh2 blocking peptide, positive staining was almost completely eliminated (Fig. 2a, right). The sensitivity and specificity shown in 1, 2 suggested strongly that this antibody could be used in immunohistochemistry. Therefore, we carried out immunohistochemical analysis in 57 HNSCC cases. In many cases, Pirh2 was strongly expressed in the nests of HNSCC and localized to the cytoplasm of cancer cells (Fig. 2b). However, the staining of Pirh2 was remarkably weak or negligible in the normal epithelium, whereas scattered fibroblasts and endothelial cells in the stroma showed faint positive staining. The mean Pirh2 LI was 63.93% (SD, 24.09; range, 11.74–99.9%) in all cancer cases and 2.46% (SD, 4.28%, range 0–12.29%) in normal squamous epithelium (Fig. 2c).
Figure 2.
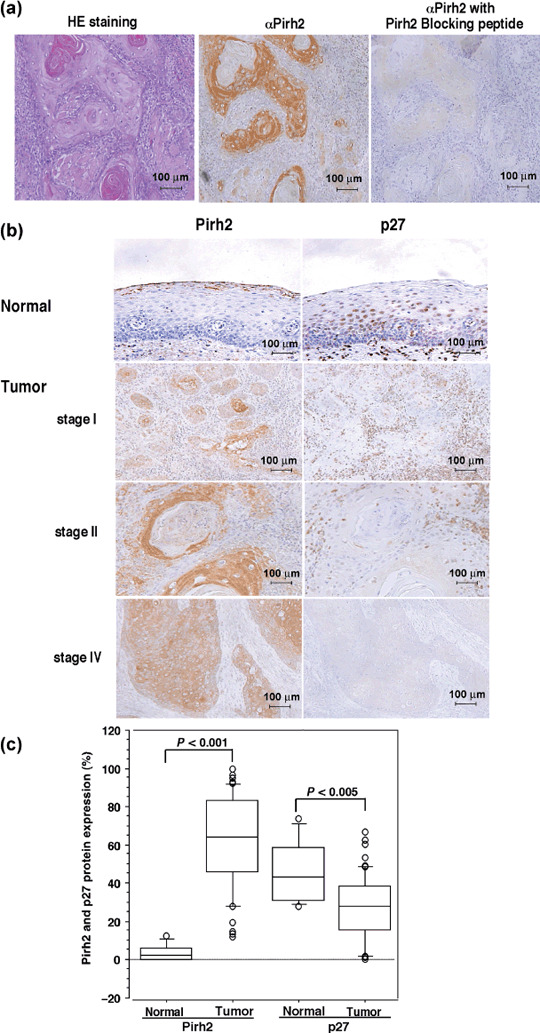
Overexpression of Pirh2 in head and neck squamous cell carcinoma (HNSCC) tissues. (a) Immunohistochemical analysis using the antigen peptide neutralized anti‐Pirh2 antibody (×200). A HNSCC specimen was subjected to immunohistochemical analysis using anti‐Pirh2 antibody preincubated with or without Pirh2 blocking peptide. (b) Expression of Pirh2 and p27 in normal epithelium and HNSCC were examined by immunohistochemical analysis (×200). Three representative HNSCC samples (stages I, II, and IV) and one normal oral epithelium are shown. (c) Box plot analysis of Pirh2 and p27. The graph shows the staining values of Pirh2 or p27 protein expression in normal oral epithelium (normal) (n = 10) and HNSCC (tumor) (n = 57). The correlation was analyzed by Mann–Whitney's U‐test. HE, hematoxylin–eosin.
In contrast, the distribution pattern and staining intensities of p27‐positive cells showed remarkable differences. p27 was expressed in the nuclei of normal epithelium (Fig. 2b). In HNSCC, the p27 staining revealed only a few positive cells in the cancer nests, whereas positive staining was observed in the stromal cells surrounding them (Fig. 2b). The mean p27 LI was 27.09% (SD, 16.97%, range, 0.00–66.64%) in cancerous samples and 43.21% (SD 16.35%, range 28.02–73.60%) in normal tissue (Fig. 2c). Thus, we concluded that Pirh2 expression was significantly increased but p27 was decreased in HNSCC compared with normal epithelium. As shown in the box plots in Figure 2(c), there was a statistically significant difference between HNSCC and the normal epithelium in the expression of both proteins (Pirh2, P < 0.001; p27, P < 0.005). These results were also confirmed using Fisher's exact test (Pirh2, P = 0.0003; p27, P = 0.033) (Table 1). In addition, there was an inverse correlation between the expression of Pirh2 and p27 proteins in HNSCC (P = 0.003) and normal epithelium (P = 0.47) using Spearman's rank correlation coefficient test.
Table 1.
Expression of Pirh2 and p27 in head and neck squamous cell carcinoma (HNSCC) and its correlation with clinicopathologic parameters
| Tissue | Expression of Pirh2 | Expression of p27 | |||||
|---|---|---|---|---|---|---|---|
| No. cases | Low (<60%) | High (≥60%) | P‐value | Low (<20%) | High (≥20%) | P‐value | |
| Normal epithelium | 10 | 10 | 0 | 0.0003** | 0 | 10 | 0.033** |
| HNSCC | 57 | 22 | 35 | 18 | 39 | ||
| Tumor size (T) | |||||||
| T1 | 20 | 11 | 9 | 0.014* | 3 | 17 | 0.013* |
| T2 | 25 | 10 | 15 | 8 | 17 | ||
| T3 | 2 | 0 | 2 | 1 | 1 | ||
| T4 | 10 | 1 | 9 | 6 | 4 | ||
| Lymph node metastasis (N) | |||||||
| Negative | 49 | 21 | 28 | 0.104** | 13 | 36 | 0.056** |
| Positive | 8 | 1 | 7 | 5 | 3 | ||
| Pathological stage (p‐Stage) | |||||||
| I | 19 | 11 | 8 | 0.006* | 3 | 16 | 0.019* |
| II | 21 | 9 | 12 | 6 | 15 | ||
| III | 2 | 0 | 2 | 1 | 1 | ||
| IV | 15 | 2 | 13 | 8 | 7 | ||
| Differentiation | |||||||
| Well | 40 | 16 | 24 | 0.695* | 9 | 31 | 0.033* |
| Moderate | 16 | 6 | 10 | 9 | 7 | ||
| Poor | 1 | 0 | 1 | 0 | 1 | ||
| p27 expression | |||||||
| Low (<20%) | 18 | 3 | 15 | 0.019** | |||
| High (≥20%) | 39 | 19 | 20 | ||||
P‐value was identified by Spearman's rank correlation coefficient test.
P‐value was identified by Fisher's exact test.
Correlation between Pirh2, p27 expression, and clinicopathological parameters. As shown in Table 1, there was a positive correlation between Pirh2 expression and tumor size (T‐factor) and pathological stage (p‐Stage), but not with lymph node metastasis (N‐factor) or differentiation (P = 0.014, 0.006, 0.104, 0.695, respectively), that is, high Pirh2 LI cases tended to have larger tumor size, and to be at an advanced disease stage. By contrast, there was an inverse correlation of p27 expression and T‐factor, p‐Stage, and differentiation, but not with N‐factor (P = 0.013, 0.019, 0.033, 0.056, respectively), that is, high p27 LI cases showed smaller tumor size, a more differentiated subtype, and were at an early disease stage (Table 1). Consistently, there was an inverse correlation between the expression of Pirh2 and p27 (P = 0.019) (Table 1).
Correlation between Pirh2 or p27 expression and survival rate. As shown in Figure 3(a), the cumulative survival rate of patients with high Pirh2 LI (≥60%) was markedly lower than that of patients with low Pirh2 LI (<60%) (P = 0.03). By contrast, the cumulative survival rate of the patients with high p27 LI (≥20%) was markedly higher than those with low p27 LI (<20%) (P = 0.02) (Fig. 3b). Next, we classified 57 patients into four groups (low Pirh2 and high p27, low Pirh2 and low p27, high Pirh2 and low p27, high Pirh2 and high p27) and evaluated the relationships with survival rate. The patients with low Pirh2 and high p27 showed the most favorable prognosis, followed by high Pirh2 and high p27, low Pirh2 and low p27, and high Pirh2 and low p27 (Fig. 3c). There was a clear difference between low Pirh2 and high p27 and high Pirh2 and low p27 with statistical significance (P = 0.004). In the univariate analysis, Pirh2 expression was well correlated with poor prognosis in HNSCC (P = 0.018) (Table 2). Moreover, multivariate analysis by the Cox proportional hazard model showed that high Pirh2 expression was an independent poor prognostic factor when compared with standard prognostic variables (P = 0.028) (Table 2). This statistical significance was also present when compared with p‐Stage and differentiation (P = 0.028) (data not shown).
Figure 3.
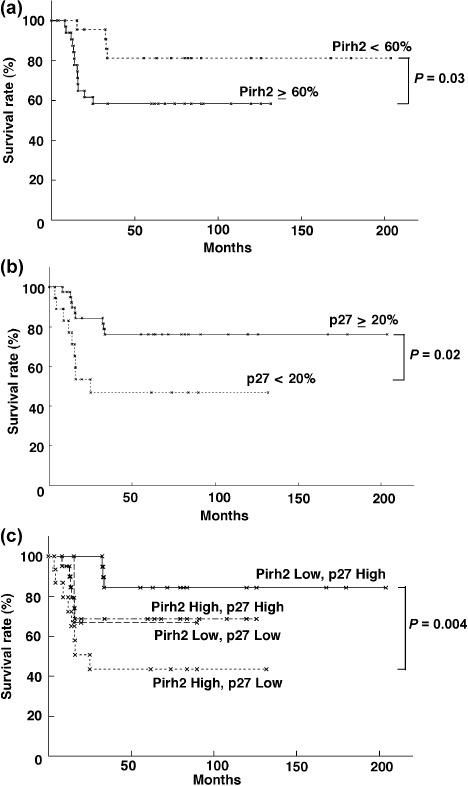
Correlation between the expression of Pirh2 and survival rate. (a) Inverse correlation between the expression of Pirh2 and survival rate. The prognosis of high expression of Pirh2 (Pirh2 ≥ 60%) (n = 35) and low expression of Pirh2 (Pirh2 < 60%) (n = 22) was examined. (b) Correlation between p27 expression and survival rate. The prognosis of high expression of p27 (p27 ≥ 20%) (n = 39) and low expression of p27 (p27 < 20%) (n = 18) was examined. (c) The relationship of Pirh2 and p27 expression to survival. Prognosis was compared among patients with Pirh2 ≥ 60% and p27 < 20% (n = 15), Pirh2 < 60% and p27 ≥ 20% (n = 19), Pirh2 ≥ 60% and p27 ≥ 20% (n = 20), and Pirh2 < 60% and p27 < 20% (n = 3). The statistical significances of these data were evaluated by the Log‐rank test.
Table 2.
Univariate and multivariate analysis of prognostic factors in 57 head and neck squamous cell carcinoma
| Factor | Relative risk | 95% confidence interval | P‐value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.015 | 0.989–1.042 | 0.249 |
| Tumor size (T) | 1.729 | 0.681–4.391 | 0.250 |
| Lymph node metastasis (N) | 4.538 | 1.850–11.131 | 0.001 |
| Pathological stage (p‐Stage) | 2.234 | 0.978–5.102 | 0.056 |
| Differentiation | 2.481 | 1.084–5.679 | 0.032 |
| Pirh2 expression | 1.026 | 1.004–1.047 | 0.018 |
| Multivariate analysis | |||
| Tumor size (T1‐2/T3‐4) | 1.036 | 0.347–3.088 | 0.950 |
| Lymph node metastasis (+/–) | 3.347 | 1.306–8.574 | 0.012 |
| Differentiation (well/moderate to poor) | 2.418 | 0.980–5.966 | 0.055 |
| Pirh2 expression | 1.028 | 1.003–1.053 | 0.028 |
Pirh2, p53‐inducible protein with RING‐H2 domain.
Discussion
It has been clarified that p27 is degraded by the ubiquitin proteasome pathway via SCF–Skp2 E3 ligase in collaboration with Cks1.( 13 , 14 ) Skp2 and Cks1 are overexpressed in several human cancers, such as those of the oral mucosa (including HNSCC), lung, breast, and others.( 6 , 15 , 16 , 17 ) Pirh2 E3 ligase also targets p27; however, to date there has been no report regarding the expression of Pirh2 in HNSCC as well as the effect of Pirh2 depletion on cell growth in HNSCC cells. In the present study, we observed that downregulation of Pirh2 expression by siRNA induced accumulation of p27 in HNSCC cells and inhibited growth in all five kinds of HNSCC cell line that were tested. These results were confirmed in other cell lines such as T98G (glioblastoma), MCF7 (breast carcinoma), Colo320DM (colon carcinoma), and HeLa cells (uterine cervical carcinoma) in our previous experiments( 8 ) (and data not shown). Overall, our results strongly suggest that Pirh2 negatively regulates p27 stability and positively regulates cell cycle progression and tumor growth.
Moreover, we showed overexpression of Pirh2 and a significant inverse correlation between Pirh2 and p27 in human HNSCC specimens. Therefore, p27 regulation is largely dependent on Pirh2 expression and activity in human malignancies, in particular in HNSCC, without any relation to other E3 ligases for p27. These results indicate that inhibition of Pirh2 expression and activity using siRNA or specific inhibitors can be a novel modality of molecularly targeted cancer therapy aimed at suppression of p27 degradation.
Pirh2 was originally identified as a p53‐inducible and p53‐interacting protein that promotes p53 ubiquitylation and degradation. Pirh2 promotes p53 ubiquitylation and represses p53 functions, including p53‐dependent transactivation and growth inhibition.( 9 ) In human cancers, although Pirh2 overexpression was shown in lung and prostate cancers by immunohistochemistry,( 18 , 19 ) there has been no study analyzing the correlation between Pirh2 expression and tumor staging or Pirh2 targets. Logan et al. reported that the expression of Pirh2 in human prostate cancer correlated with Gleason score and bone metastasis but did not correlate with prostate‐specific antigen levels, survival, or androgen dependency.( 19 ) p53, another target of Pirh2, is frequently mutated in various cancers, and mutant p53 shows oncogenic activity and promotes carcinogenesis.( 9 ) Strangely, not only wild‐type p53 but also mutant p53 (273H) has been shown to be targeted by Pirh2.( 18 ) These evidences are contradictory, because degradation of mutant p53 may be involved in tumor suppression. Therefore, further studies are required. In contrast to p53, p27 mutations in human tumors are extremely rare.( 20 ) Although several targets for Pirh2 have been reported, such as p53, androgen receptor, ɛ‐COP (epsilon‐subunit of coatmer complex), and SR‐β (signal recognition particle receptor beta subunit),( 9 , 21 , 22 , 23 ) because p27 level reverse‐correlates with Pirh2 expression, p27 may be an important target of Pirh2 in human cancers.
In the present study, we found remarkable differences between the expression of Pirh2 in HNSCC and that in normal oral mucosa. Furthermore, high expression of Pirh2 in cancer was associated with low expression of p27, and was well correlated with T‐factor, p‐Stage, and poor prognosis in HNSCC patients. In particular, the enlargement of tumor mass may be partially because of enhanced cell growth mediated by p27 degradation by Pirh2. Our report is the first to show a correlation between clinical parameters (T‐factor, p‐Stage) and Pirh2 expression, and a reverse correlation between p27 level and the expression of Pirh2 in human tumors.
There was a statistically significant correlation between survival rate and individual expression of Pirh2 or p27. Furthermore, expression of Pirh2 and p27 showed an inverse correlation in HNSCC. As a result, high expression of Pirh2 and low expression of p27 was significantly correlated with poor prognosis. The survival rate of patients with high expression of Pirh2 (≥60%) was lower than other patients, suggesting that the expression level of Pirh2 may have an effect on survival rate as well as that of p27. Moreover, a multivariate analysis revealed that high Pirh2 expression was an independent variable that correlated with shorter survival.
In conclusion, we have shown that Pirh2 overexpression was well correlated with poor prognosis, at least partially through degradation of p27. Pirh2 has a role as an essential factor decreasing p27 in human HNSCC. Moreover, Pirh2 is a possible candidate as a molecular target for cancer therapy.
Supporting information
Fig. S1. Expression of p53‐inducible protein with RING‐H2 domain (Pirh2) and p27 protein in five head and neck squamous cell carcinoma cell lines.
Fig. S2. Cell cycle profiles of Ho‐1‐u‐1 and Ho‐1‐N‐1 cell lines transfected with control or p53‐inducible protein with RING‐H2 domain (Pirh2) short interfering RNA.
Table S1. Patient characteristics
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
We thank Dr Tatsuo Yamamoto for useful discussion and Dr Sayuri Suzuki for both useful discussion and technical assistance. This work was supported in part by grants from the Ministry of Education, Science, Sports, Culture, and Technology of Japan (M.K. (19057005, B19370083), Y.D. (C20590351) and K.K. (C20012022)), and by a COE program of the Hamamatsu University School of Medicine from the Ministry of Education, Science, Sports, Culture, and Technology of Japan (M.K.).
References
- 1. Zhang H, Sun XF. Loss of p27 expression predicts poor prognosis in patients with Dukes’ B stage or proximal colorectal cancer. Int J Oncol 2001; 19: 49–52. [PubMed] [Google Scholar]
- 2. Tan P, Cady B, Wanner M et al . The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997; 57: 1259–63. [PubMed] [Google Scholar]
- 3. Ishihara S, Minato K, Hoshino H et al . The cyclin‐dependent kinase inhibitor p27 as a prognostic factor in advanced non‐small cell lung cancer: its immunohistochemical evaluation using biopsy specimens. Lung Cancer 1999; 26: 187–94. [DOI] [PubMed] [Google Scholar]
- 4. Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, Okayasu I. Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am J Pathol 2000; 156: 585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mineta H, Miura K, Suzuki I et al . Low p27 expression correlates with poor prognosis for patients with oral tongue squamous cell carcinoma. Cancer 1999; 85: 1011–17. [DOI] [PubMed] [Google Scholar]
- 6. Inui N, Kitagawa K, Miwa S et al . High expression of Cks1 in human non‐small cell lung carcinomas. Biochem Biophys Res Commun 2003; 303: 978–84. [DOI] [PubMed] [Google Scholar]
- 7. Kitajima S, Kudo Y, Ogawa I et al . Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am J Pathol 2004; 165: 2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hattori T, Isobe T, Abe K et al . Pirh2 promotes ubiquitin‐dependent degradation of the cyclin dependent kinase inhibitor p27Kip1. Cancer Res 2007; 67: 10789–95. [DOI] [PubMed] [Google Scholar]
- 9. Leng RP, Lin Y, Ma W et al . Pirh2, a p53‐induced ubiquitin‐protein ligase, promotes p53 degradation. Cell 2003; 112: 779–91. [DOI] [PubMed] [Google Scholar]
- 10. Leon B, John WE, Peter R, David S. WHO Classification of Tumors, Pathology and Genetics Head and Neck Tumors. Lyon: International Agency for Research on Cancer, 2005. [Google Scholar]
- 11. Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumors (UICC), 5th edn. New York: John Wiley and Sons, 1997. [Google Scholar]
- 12. Kudo Y, Takata T, Ogawa I et al . Reduced expression of p27Kip1 correlates with an early stage of cancer invasion in oral squamous cell carcinoma. Cancer Lett 2000; 151: 217–22. [DOI] [PubMed] [Google Scholar]
- 13. Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27Kip1 ubiquitination and degradation is regurated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9: 661–4. [DOI] [PubMed] [Google Scholar]
- 14. Ganoth D, Bornstein G, Ko TK et al . The cell‐cycle regulatory protein Cks1 is required for SCFSkp2‐mediated ubiquitinylation of p27. Nat Cell Biol 2001; 3: 321–4. [DOI] [PubMed] [Google Scholar]
- 15. Gstaiger M, Jordan R, Lim M et al . Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 2001; 98: 5043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuda T, Inoue H, Sonoda H et al . Clinical and biological significance of S‐phase kinase‐associated protein2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res 2002; 62: 3819–25. [PubMed] [Google Scholar]
- 17. Slotky M, Shapira M, Ben‐Izhak O et al . The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res 2005; 7: 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duan W, Gao L, Druhan LJ et al . Expression of Pirh2, a newly identified ubiquitin protein ligase, in lung cancer. J Natl Cancer Inst 2004; 96: 1718–21. [DOI] [PubMed] [Google Scholar]
- 19. Logan IR, Gaughan L, McCracken SR, Sapointzi V, Leung HY, Robson CN. Human Pirh2 enhances androgen receptor signaling through inhibition of histone deacetylase1 and is overexpressed in prostate cancer. Mol Cellular Biol 2006; 26: 6502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawamata N, Morosetti R, Miller CW et al . Molecular analysis of the cyclin‐dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 1995; 55: 2266–9. [PubMed] [Google Scholar]
- 21. Beitel LK, Elhaji YA, Lumbroso R et al . Cloning and characterization of an androgen receptor N‐terminal‐interacting protein with ubiquitin‐protein ligase activity. J Mol Endocrinol 2002; 29: 41–60. [DOI] [PubMed] [Google Scholar]
- 22. Maruyama S, Miyajima N, Bohgaki M et al . Ubiquitylation of ɛ‐COP by PIRH2 and regulation of the secretion of PSA. Mol Cell Biochem 2008; 307: 73–82. [DOI] [PubMed] [Google Scholar]
- 23. Abe K, Hattori T, Isobe T et al . Pirh2 interacts with and ubiquitylates signal recognition particle receptor beta subunit. Biomed Res 2008; 29: 53–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of p53‐inducible protein with RING‐H2 domain (Pirh2) and p27 protein in five head and neck squamous cell carcinoma cell lines.
Fig. S2. Cell cycle profiles of Ho‐1‐u‐1 and Ho‐1‐N‐1 cell lines transfected with control or p53‐inducible protein with RING‐H2 domain (Pirh2) short interfering RNA.
Table S1. Patient characteristics
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


