Figure 1.
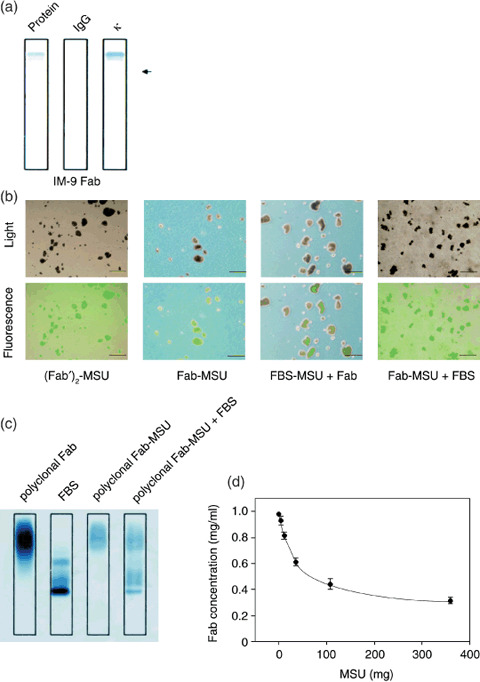
Monosodium urate (MSU) crystals bound with Fab fragments. (A) Purification and positive charge of Fab fragments from IM‐9 supernatants were confirmed using immunofixation electrophoresis; protein fractionation using sulfosalicylic acid and acetic acid (Protein), fixed using antihuman immunoglobulin G antibody (IgG), or fixed using antihuman κ antibody (κ). The arrow shows the position of sample application. (B) Fluorescein isothiocyanate (FITC)‐labeled (Fab′)2 attached to MSU crystals ([Fab′]2‐MSU), FITC‐labeled Fab attached to MSU crystals (Fab‐MSU), fetal bovine serum attached to MSU crystals followed by FITC‐labeled Fab (FBS‐MSU + Fab), or FITC‐labeled Fab attached to MSU crystals followed by FBS (MSU‐Fab + FBS) were examined by light microscopy and fluorescence microscopy. Scale lines, 10 µm. (C) Proteins attached to MSU crystals were investigated by immunofixation electrophoresis. MSU and polyclonal Fab fragments from healthy volunteers were incubated at 4°C for 3 h (polyclonal Fab‐MSU), and then some of polyclonal Fab‐MSU complexes were mixed with 10% FBS for 3 h (polyclonal Fab‐MSU + FBS). After dissolving MSU crystals, the solution was concentrated and applied to immunofixation electrophoresis. (D) Fab concentration in supernatant containing a fixed amount of Fab (100 µg) and various amounts of MSU crystals were measured in order to calculate the amount of attached Fab fragments. Data are expressed as mean ± standard deviation of four independent experiments.
