Figure 1.
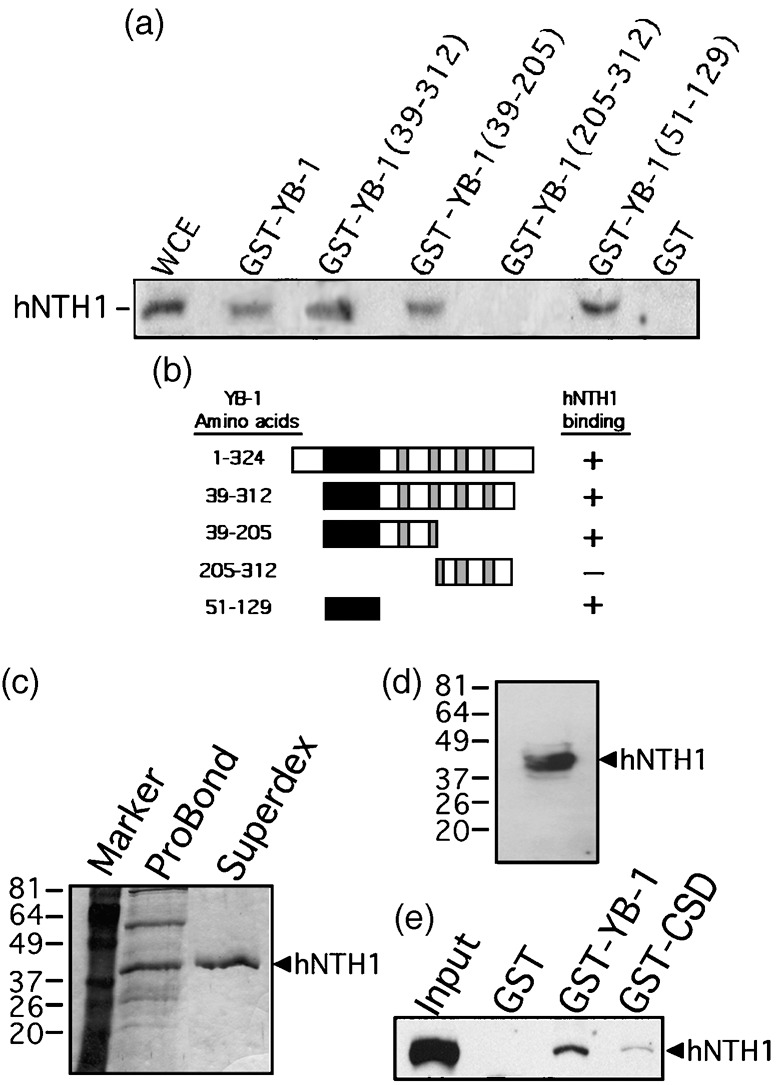
Interaction of different domains of Y‐box‐binding protein‐1 (YB‐1) with human endonuclease III (hNTH1) in total cell extract. (a) Immunoblot against hNTH1 proteins bound to different GST‐YB‐1 affinity Sepahrose beads. Human MCF7 whole cell extracts (WCE) were incubated with either 50 µg of GST‐YB‐1 or GST‐linked glutathione‐sepharose beads overnight. Proteins bound to the affinity beads were analyzed by SDS‐PAGE with antibodies against hNTH1. (b) Schematic representation of different YB‐1 polypeptides that were used in the YB‐1 affinity chromatography experiments. The black box is the cold shock domain and the gray boxes are the basic/acidic cluster domains. The amino acid residues of the YB‐1 fragments used in this study are indicated on the left. hNTH1 binding is indicated on the right by the ‘+’ sign. The ‘–’ sign indicates no binding detected. (c) Coomassie staining of a gel containing purified hNTH1 after the nickel column step (ProBond resin) and after fractionation on Superdex‐200 (fraction number 29). The molecular weight, in kDa, is indicated on the left. (d) Western blot of purified hNTH1 after the final Superdex‐200 step (fraction 29) with an antibody against hNTH1. (e) Interaction of purified hNTH1 (after the final Superdex‐200 step) with 50 µg of GST‐YB‐1, GST‐CSD (cold shock domain of YB‐1; amino acids 51–129), or GST‐linked glutathione‐sepharose beads.
