Figure 3.
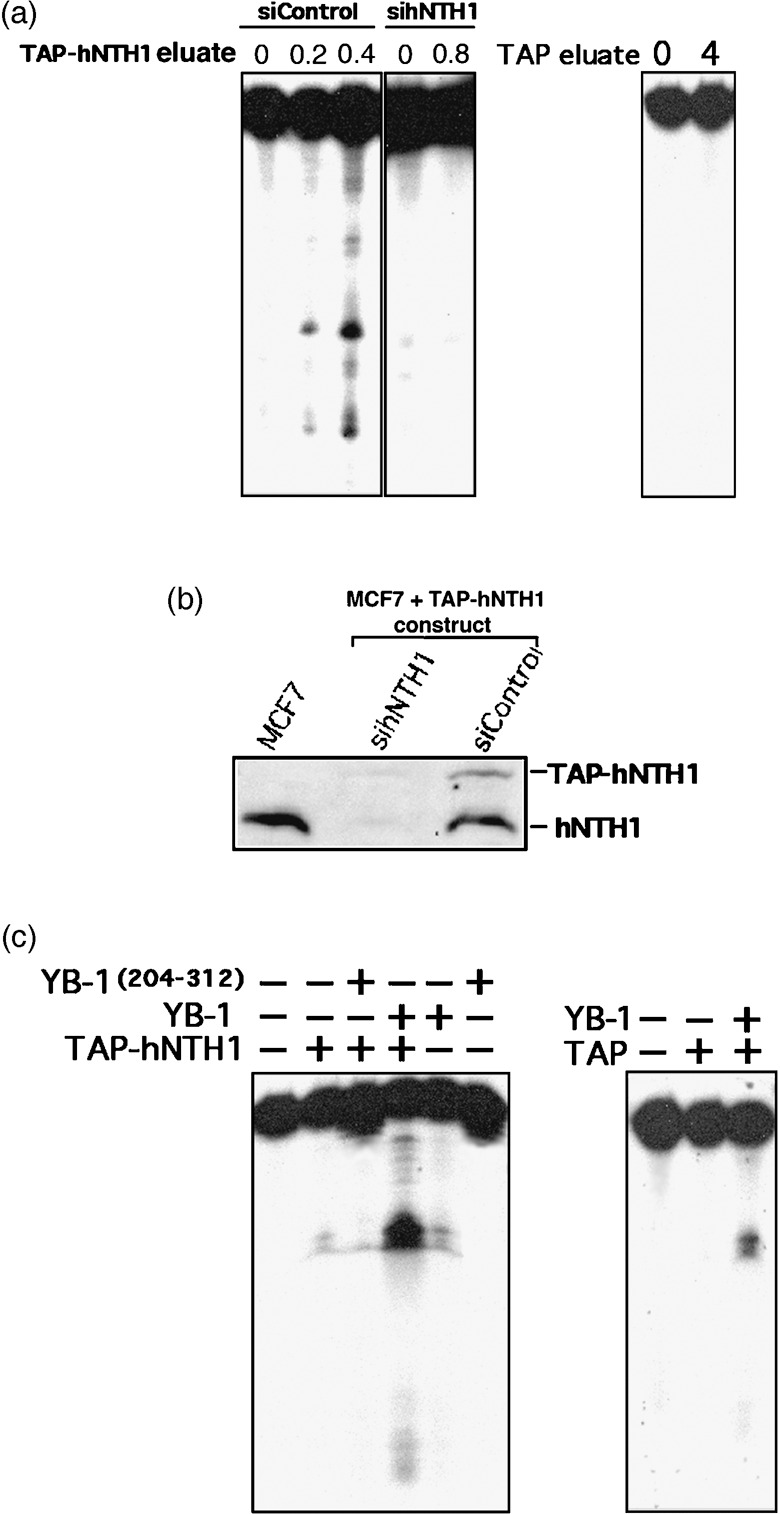
Nuclease activity of eluted TAP‐hNTH1 in the presence of purified Y‐box‐binding protein‐1 (YB‐1). (a) Tap‐hNTH1 MCF7 expressing cells were transfected with either siRNA control (siControl) or siRNA against human endonuclease III (hNTH1) (panels on the left). Two days later the TAP‐hNTH1 complex was purified on streptavidin column and eluted with biotin. Eluted TAP‐hNTH1 proteins (0, 0.2, or 0.4 µg) were incubated for 30 min at 37°C as described in ‘Materials and Methods’ with a radioactive DNA duplex containing 8‐oxoguanine residues. Reactions were stopped in the appropriate dye buffer and cleaved DNA products were analyzed on 14% denaturing polyacrylamide gels. The panel on the right represents nuclease assays with or without eluate from MCF7 cells containing an empty TAP vector (0 or 0.8 µg). (b) Western blot showing intracellular levels of endogenous hNTH1 and TAP‐hNTH1 after transfection with a siRNA specific to hNTH1 or with a scrambled control siRNA. The first lane contained whole lysate from untransfected parental MCF7 cells. Each lane contains 75 µg of whole cell lysate. (c) Nuclease activity with TAP‐hNTH1 in the absence or presence of either a full length YB‐1 peptide (2 µg) or a purified peptide containing amino acids 205–312 of YB‐1 (2 µg). (Panel on the left.) Note that YB‐1(205–312) does not bind to hNTH1. The panel on the right represents nuclease assays with eluate from MCF7 cells containing an empty TAP vector (0.8 µg) in the presence or absence of YB‐1 (2 µg).
