Abstract
Expression levels of p27kip1, a negative regulator of the G1 phase of the cell cycle, and 8‐hydroxydeoxyguanosine (8‐OHdG), a marker of oxidative DNA damage, were assessed by immunostaining in a series of renal cell carcinomas (RCCs) and their prognostic significance was evaluated. Expression of p27kip1 as well as of the α‐subunit of the dystroglycan (DG) complex, previously reported to be altered in RCC, was also evaluated by western blot analysis. Nuclear expression of p27kip1 was reduced in a significant fraction of tumors and low p27kip1 staining correlated with higher tumor grade (P < 0.01). Recurrence and death from clear cell RCCs were significantly more frequent in p27kip1‐low expressing tumors and Kaplan–Meier curves showed a significant separation between high vs low expressor groups for both disease‐free (P = 0.011) and overall (P = 0.002) survival. Low nuclear expression of p27kip1 as well as loss of α‐DG were confirmed to be independent prognostic parameters at a multivariate analysis and the simultaneous loss of both molecules defined a “high‐risk” group of patients with increased risk of recurrence (RR = 28.7; P = 0.01) and death (RR = 12.9; P = 0.03). No significant correlation with clinical or pathological parameters was found for 8‐OHdG staining. Western blot analyses suggested a post‐translational mechanism for the loss of α‐DG expression and demonstrated that cytoplasmic dislocation of the protein contributes to the loss of active nuclear p27kip1. Loss of nuclear p27kip1 is a frequent event in human RCCs and is a powerful predictor of poor outcome which, in combination with low DG expression, could help to identify high‐risk patients with clear cell RCC. (Cancer Sci 2010; 00: 000–000)
Renal cell carcinoma (RCC) represents a major health challenge worldwide since it accounts for about 2% of all cancers in industrialized countries with a steadily rising incidence and mortality in the USA and in other countries in the past three decades.( 1 ) Renal cell carcinoma (RCC) is a clinicopathologically heterogeneous disease, encompassing several histological subtypes( 1 , 2 , 3 ) with clear‐cell RCC being the most frequent (up to 80%) and responsible for the majority of deaths.( 1 ) Renal cell carcinoma (RCC) is characterized by an unpredictable clinical behavior ranging from an aggressive presentation with rapid disease progression until death to a mild course with long‐term survival. Available prognostic indicators (i.e. tumor stage and grade) are unable to accurately predict outcome especially in clear‐cell RCC patients undergoing radical surgery (stage I–III). We recently reported that loss of the dystroglycan (DG) complex, a ubiquitous non‐integrin adhesion molecule, is a frequent event in RCC and displays a prognostic significance in RCC patients being associated with a poor prognosis.( 4 ) Deregulation of the normal cell cycle is a frequent event in human tumors and plays an important role in malignant transformation. p27Kip1 is a negative regulator of the G1 phase of the cell cycle, is regarded as a tumor suppressor gene, and is frequently lost in tumor cells.( 5 ) This protein is frequently deregulated in RCC( 6 , 7 ) but whether its expression can be used as a prognostic indicator remains to be clarified.
The reactive oxygen species formed during exposure to environmental oxidants and during endogenous metabolic processes play an important role in the pathogenesis of cancer and other degenerative diseases. Reactive oxygen species can induce genotoxic damage, and oxidation of the C8 of guanine is one of the most abundant types of oxidative DNA damage. The modified base 8‐hydroxydeoxyguanosine (8‐OHdG) is a mutagenic lesion in vivo and in vitro, leading to G–T and A–C substitutions.( 8 ) It is therefore suggested as a sensitive biomarker for molecular epidemiological assessment of oxidative stress. An immunoperoxidase method for 8‐OHdG detection in single cells has been developed using a specific anti‐8‐OHdG monoclonal antibody.( 9 ) We previously optimized the method for the evaluation of oxidative DNA damage in human cells and have successfully applied this technique to detect 8‐OHdG levels in human tissues.( 10 )
In this study, the expression of p27Kip1 and the extent of endogenous oxidative DNA damage were evaluated in a series of RCCs and their prognostic significance was investigated in the subset of clear cell RCC.
Materials and Methods
Samples. Tissue specimens used for immunohistochemical analyses have been previously described( 4 ) and were obtained from 125 consecutive unselected Italian patients with renal epithelial tumors who underwent surgery at the “Versilia” Hospital of Lido di Camaiore during the period 2002–2005. No familiar cases were included in the series of patients analyzed in the study and no patient received preoperative treatments. Formalin‐fixed, paraffin‐embedded specimens were retrieved for this study from the archives of the Department of Pathology and two experienced pathologists (A.S. and F.D.L.) confirmed the histological diagnosis of each lesion. The selection did not require approval by an Institutional Review Board because the samples were coded and the names of the patients were not revealed. All cases were classified according to World Health Organization (WHO) criteria( 3 ) and graded in accordance with the Fuhrman nuclear grading system.( 11 ) Tumor stage was defined according to the International Union Against Cancer (IUCC) 2002 TNM classification.( 2 )
Immunoperoxidase detection of p27kip1 and of 8‐OHdG. All immunohistochemical analyses were performed on routinely processed, formalin‐fixed, paraffin‐embedded tissues employing an avidin–biotin complex immunoperoxidase technique, as previously described.( 10 , 12 , 13 ) Briefly, for detection of p27kip1, sections were dewaxed, rehydrated and then microwave pre‐treated (1 mM EDTA buffer, pH 8.0) for antigen retrieval. A polyclonal anti‐p27kip1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a concentration of 1 μg/mL (in PBS with 10% goat serum). A breast carcinoma with known positive immunostaining for p27kip1 served as a positive control. A strong nuclear staining of lymphocytes provided a useful internal positive control for preservation of the p27kip1 immunogenicity in most sections examined. Only a clear nuclear staining was regarded as positive. All scoring and interpretations of the results were made by two of the authors independently (M.M. and F.D.L.) without knowledge of other clinicopathological variables. The few cases with discrepant scoring were re‐evaluated jointly on a second occasion, and agreement was reached. Detection of the α‐DG subunit of the dystroglycan (DG) complex has been previously reported.( 4 ) Immunohistochemical detection of 8‐OHdG was performed as previously described.( 10 ) The results obtained were standardized to controls and stratified into three categories in term of staining intensity, as previously reported. Briefly, semi‐quantitative evaluation of the staining was performed measuring the relative intensity of nuclear staining in 100 randomly selected cells in the areas of interest, excluding stromal and inflammatory cells. The image was obtained in black and white and the average optical density was recorded. The results obtained were standardized to controls and stratified into three categories in term of staining intensity: 1 = weak; 2 = moderate; and 3 = strong. Samples were also stratified in term of percentage of positive cells as: 0 = 0%; 1 = 1–10%; 2 = 11–50%; and 3 = >50% positive cells. A staining score was defined taking into account both intensity of staining and percentage of positive cells and was calculated as: % positive cells × staining intensity, and was stratified as: negative = 0; low = 1; moderate = 2–3; and strong >3.( 10 , 14 )
Total protein extraction and western blot analysis. Samples used for western blot analyses were from patients who underwent surgery in our institution in the period from January 2008 to June 2009. No patient received preoperative chemotherapy or radiotherapy. Briefly, after obtaining informed consent from patients, when possible a fragment of surgical specimen was snap‐frozen in liquid nitrogen immediately after surgery and stored at −80°C until use. For renal normal tissue samples, biopsies were taken where the tissue appeared free from cancer not contiguous to resection site. Total protein extracts and nuclear and cytoplasmic fractions were prepared as described.( 15 , 16 ) The polyclonal anti‐p27kip1 antibody (Santa Cruz Biotechnology) was used diluted at 1:100. The monoclonal antibody to α‐DG (clone VIA4‐1) was obtained from Upstate Biotechnology (Lake Placid, NY, USA) and was used diluted at 1:2000. The monoclonal antibody to β‐DG (clone 43DAG/8D5) was from Novocastra (Newcastle, UK) and was used diluted at 1:50. Protein extraction was independently performed twice for each sample. Similar results were obtained when the two protein extracts from each sample were independently tested.
Statistical analysis. The association between p27kip1 and 8‐OHdG expression and other molecular and clinicopathological parameters were calculated using contingency table methods and tested for significance using the Pearson’s chi‐squared test. Follow‐up data were available for 68 clear‐cell RCC patients since the remainder were referred to other institutions after surgery. Patients followed at our institution were uniformly followed and disease‐free survival (DFS) was defined as the interval between surgery and the first documented evidence of disease in the local‐regional area and/or distant sites. Overall survival was defined as the interval between surgery and death from the disease. Patients who died of causes unrelated to the disease were not included in the survival analyses. Disease‐free and overall survival curves were calculated using the Kaplan–Meier method and the log‐rank test was used to compare survival curves. Univariate and multivariate relative risks were calculated using Cox proportional hazards regression. All calculations were performed using the STATA statistical software package (Stata, College Station, TX, USA) and the results were considered statistically significant when the P‐value was ≤0.05.
Results
p27kip1 expression is reduced in human renal cell carcinomas and correlates with the clinical outcome of patients. The expression of p27kip1 and 8‐OHdG was evaluated by immunostaining in a series of 125 primary human renal epithelial cancers (Table 1). For both parameters, only a clear nuclear staining was regarded as positive. Normal renal parenchyma was present in about half of the cases and a positive nuclear staining for p27kip1 was always observed both at the tubular and glomerular level (Fig. 1a and data not shown). Interestingly, a mild cytoplasmic staining was sometimes observed both in normal and cancer renal tissues and tended to be more evident in tumor cells independently of nuclear staining (Fig. 1a–c and data not shown).
Table 1.
Clinicopathological features of renal epithelial tumors used for immunohistochemical (a) and western blot (b) analyses
| (a) | |
| Number of patients | 125 |
| Age (years) | |
| Median (range) | 64 (23–86) |
| Gender (M/F) | 87/38 |
| Histotype | |
| Clear cell | 100 (80%) |
| Papillary | 9 (7.2%) |
| Chromophobe | 3 (2.4%) |
| Oncocytoma | 13 (10.4%) |
| Tumor stage*† | |
| I | 81 (72%) |
| II | 17 (15%) |
| III | 11 (10%) |
| IV | 3 (3.0%) |
| Grading*‡ | |
| 1 | 21 (19%) |
| 2 | 66 (59%) |
| 3 | 23 (20%) |
| 4 | 2 (2%) |
| (b) | |
| Number of patients | 34 |
| Age (years) | |
| Median (range) | 72 (36–81) |
| Histotype | |
| Clear cell | 28 (82.3%) |
| Papillary | 5 (14.7%) |
| Chromophobe | 1 (3.0%) |
| Tumor stage*† | |
| I | 28 (90.3%) |
| II | 3 (9.7%) |
| III | 0 (0%) |
| IV | 0 (0%) |
| Grading*† | |
| 1 | 4 (13%) |
| 2 | 18 (58%) |
| 3 | 7 (23%) |
| 4 | 2 (6%) |
*Oncocytomas were excluded. †According to International Union Against Cancer (UICC) TNM classification of malignant tumours, 6th edn 2002. ‡According to Fuhrman classification.
Figure 1.
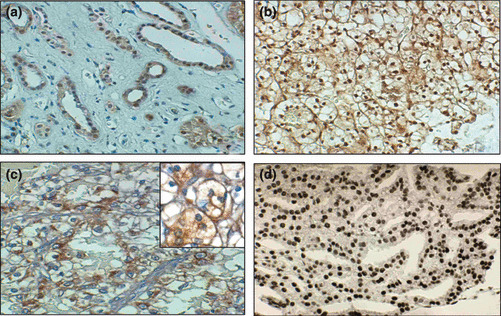
Examples of p27kip1 immunohistochemical staining in renal epithelial tumors. (a) Normal renal tissue showing nuclear staining of tubular epithelial cells. (b) Example of a well differentiated clear‐cell renal cell carcinoma (RCC) displaying a strong staining for p27kip1. (c) Example of a poorly differentiated clear‐cell RCC displaying a negative nuclear staining for p27kip1 with an evident cytoplasmic positivity (d) Example of immunostaining for 8‐hydroxydeoxyguanosine (8‐OHdG) in a representative case of clear cell RCC displaying a positive staining. Original magnifications: (a) ×200; (b) ×150; (c) ×250; (d) ×200; inset in (c) ×800.
In RCCs (oncocytomas excluded, n = 112) the median percentage of positive cells was 20% (range, 0–60; mean, 20.6%) and p27kip1 staining was not detectable in tumor cells in 51 out of 112 (45.5%) specimens despite the fact that, when present, normal renal tissue stained positively. p27kip1 staining displayed an inverse correlation with tumor grade. In fact, when cases were stratified according to tumor grade, the mean percentage of positive cells was 23 (range, 0–60; median, 45%), and 11.8 (range 0–40; median, 0) in G1/2 and G3/4 tumors, respectively, and this difference was statistically significant (P = 0.017). Moreover, using the 20% positive cells as cut‐off to distinguish between high (>20%) and low (≤20%) staining, high p27kip1 staining was detected in 51 (59%) of the 87 well/moderately differentiated tumors and in four (16%) of the less differentiated (G3/4) ones, and cross‐tab analysis confirmed a significant inverse correlation (P < 0.001) between the two parameters (Table 2). No correlation was observed with either pT parameter, tumor stage, and DG expression (Table 2).
Table 2.
p27kip1 expression in relation to clinicopathological parameters in a series of 112 RCC patients
| Total | Low n (%) | High n (%) | P‐value | |
|---|---|---|---|---|
| Age (years) | ||||
| ≤64 | 53 | 29 (55) | 24 (45) | n.s. |
| >64 | 59 | 28 (47) | 31 (53) | |
| DG | ||||
| Low | 60 | 34 (57) | 26 (43) | n.s. |
| High | 52 | 23 (44) | 29 (56) | |
| pT parameter* | ||||
| pT1 | 84 | 39 (46) | 45 (54) | n.s. |
| pT2–4 | 28 | 18 (64) | 10 (36) | |
| Stage* | ||||
| I | 81 | 36 (44) | 45 (56) | n.s. |
| II–IV | 31 | 21 (67) | 10 (33) | |
| Grading† | ||||
| 1–2 | 87 | 36 (41) | 51 (59) | <0.001 |
| 3–4 | 25 | 21 (84) | 4 (16) | |
| 8‐OHdG | ||||
| Neg.–moderate | 83 | 31 (37) | 52 (63) | n.s. |
| Strong | 29 | 9 (31) | 20 (69) | |
*According to International Union Against Cancer (UICC) TNM classification of malignant tumours, 6th edn 2002. †According to Fuhrman classification. 8‐OHdG, 8‐hydroxydeoxyguanosine; DG, dystroglycan; n.s., not significant; RCC, renal cell carcinoma.
To study a more homogeneous group of patients, the relationship between p27kip1 staining and clinical outcome was analyzed within the subset of patients with clear‐cell RCC (n = 68) for which follow‐up data were available (mean, 29; range, 4–104 months). The median percentage of positive cells was 20% (range, 0–60; mean, 18.7%) in this subset of cancers. Both disease recurrence and disease‐related death were more frequent in patients whose tumor expressed a reduced nuclear staining for p27kip1. Overall, the median percentage of positive cells was 20 (range, 0–60; mean, 22.6 ± 19.1%) and 0 (range, 0–50; mean, 10.5 ± 18%) in non‐recurrent and recurrent cases, respectively, and this difference was significant (P = 0.017). The median DFS of p27kip1 low expressor tumors was shorter compared to high expressor cases (20.5 ± 11.8 vs 27.3 ± 15.9 months) and this difference was significant (P = 0.05). Moreover, when tumors were stratified according to p27kip1 expression, 16 (51.6%) out of 31 low expressor cases recurred during the period of follow‐up while only 10 (27%) recurred among the remaining 37 cases and this difference was significant (P = 0.008) as also confirmed by the Kaplan–Meier curves of DFS which displayed a significant separation between the two groups of patients (P = 0.011 by log‐rank test) (Fig. 2a). Similarly, 15 (48.4%) out of 31 patients with low expresssor tumors and only seven (18.9%) of the 37 remaining ones died of disease during the follow‐up period and this difference was significant (P = 0.007). Moreover, the median percentage of positive cells was 20 (range, 0–60; mean, 22.4 ± 19.2%) and 0 (range, 0–50; mean, 9.3 ± 17.3%) in alive and dead patients, respectively, and this difference was significant (P = 0.013). Thus, patients with tumors displaying a reduced staining for p27kip1 were more likely to die of the disease compared with high expressor tumors as confirmed by the Kaplan–Meier curves which displayed a significant separation between the two groups of patients (P = 0.002 by log‐rank test) (Fig. 2b). Hence, reduced expression of p27kip1 was associated with an increased risk of recurrence and death in our series of clear‐cell RCCs (Fig. 2a,b).
Figure 2.
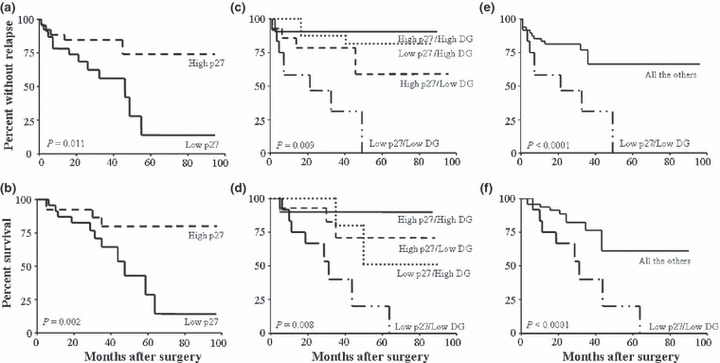
Kaplan–Meier curves for disease‐free (upper panel) and overall (lower panel) survival in a series of 60 clear‐cell renal cell carcinoma (RCC) patients. Patients were stratified by p27kip1 expression (a,b) or according to the level of p27kip1 and α‐dystroglycan (α‐DG) complex expression (c–f) (see text for details).
In a multivariate analysis performed by building a Cox hazards model that included tumor grade, tumor size, and p27kip1 staining, reduced p27kip1 staining appeared to be the only independent predictor of shorter DFS (RR = 4.326; P = 0.014; CI, 1.344–13.921) and OS (RR = 4.915; P = 0.012; CI, 1.406–17.185) (data not shown).
8‐Hydroxydeoxyguanosine (8‐OHdG) immunostaining does not correlate with any other clinicopathological parameters in human renal cell carcinomas. 8‐Hydroxydeoxyguanosine (8‐OHdG) expression was assessed by immunostaining in the same series of RCCs samples using the specific anti‐8‐OHdG monoclonal antibody 1F7( 9 , 17 , 18 ) (Fig. 1d). No staining was observed in the majority of normal specimens and in oncocytomas (data not shown). Staining was highly heterogeneous in the remaining 112 RCCs, both in terms of percent of positive cells and intensity (not shown), with the median percentage of positive cells being 0% (range, 0–60; mean, 5.7%) and the majority of tumor samples (86 of 112) showing no staining. 8‐Hydroxydeoxyguanosine (8‐OHdG) levels did not correlate with any of the analyzed parameters (grading, p27kip1, pT parameter, tumor stage, and α‐DG) (data not shown). 8‐Hydroxydeoxyguanosine (8‐OHdG) expression also did not correlate with any of the analyzed parameters and with the clinical outcome of the patients within the group of clear‐cell RCCs both in term of DFS and OS (data not shown).
Prognostic significance of combined p27kip1 and dystroglycan staining. We previously reported that α‐DG expression is an important predictor of clinical outcome in clear cell RCC patients.( 4 ) Given the lack of correlation between p27kip1 and α‐DG (Table 2), we built a second Cox regression model including both parameters with tumor grade and tumor size. Both parameters confirmed to be independent predictor of DFS (Table 3) and OS (Table 4). Moreover, when they were analyzed together, reduced expression of both p27kip1 and α‐DG demonstrated to be a powerful negative prognostic factor for both DFS (RR = 28.694; P = 0.01; confidence interval [CI], 2.169–79.460) and OS (RR = 12.947; P = 0.032; CI, 1.231–46.090), stronger than tumor grade and tumor size (5, 6).
Table 3.
Contribution of various potential prognostic factors to disease‐free survival by Cox regression analysis in a series of clear‐cell RCC patients
| Variable | Risk ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Tumor size* | 3.671 | 0.786–17.138 | 0.09 n.s. |
| Grading† | 1.011 | 0.225–4.533 | 0.98 n.s. |
| Dystroglycan‡ | 4.330 | 1.148–16.365 | 0.03 |
| p27kip1§ | 4.915 | 1.406–17.185 | 0.012 |
*Risk ratio given as pT2–4 vs pT1 tumors; †risk ratio given as G3–4 vs G1–2 tumors; ‡risk ratio given as dystroglycan (DG) low vs high‐expressor tumors; §risk ratio is given as p27kip1 low vs high‐expressor tumors. n.s., not significant, RCC, renal cell carcinoma.
Table 4.
Contribution of various potential prognostic factors to overall survival by Cox regression analysis in a series of clear‐cell RCC patients
| Variable | Risk ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Tumor size* | 2.737 | 0.648–11.551 | 0.17 n.s. |
| Grading† | 1.396 | 0.212–2.210 | 0.64 n.s. |
| Dystroglycan‡ | 5.373 | 1.400–20.610 | 0.014 |
| p27kip1§ | 4.326 | 1.344–13.921 | 0.014 |
*Risk ratio given as pT2–4 vs pT1 tumors; †risk ratio given as G3–4 vs G1–2 tumors; ‡risk ratio given as dystroglycan (DG) low vs high‐expressor tumors; §risk ratio is given as p27kip1 low vs high‐expressor tumors. n.s., not significant, RCC, renal cell carcinoma.
Table 5.
Prognostic significance of the high‐risk profile (low p27/low DG) for disease‐free survival by Cox regression analysis in clear‐cell RCC patients
| Variable | Risk | 95% confidence interval | P‐value |
|---|---|---|---|
| Tumor size* | 3.167 | 0.249–40.167 | 0.37 n.s. |
| Grading† | 3.405 | 0.345–33.524 | 0.29 n.s. |
| DG + p27kip1‡ | 28.694 | 2.169–79.460 | 0.01 |
*Risk ratio given as pT2–4 vs pT1 tumors; †risk ratio given as G3–4 vs G1–2 tumors; ‡risk ratio is given as dystroglycan (DG) and p27kip1 low expressor tumors vs the remaining ones. n.s., not significant, RCC, renal cell carcinoma.
Table 6.
Prognostic significance of the high‐risk profile (low p27/low DG) for disease‐free survival by Cox regression analysis in clear‐cell RCC patients
| Variable | Risk ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Tumor size* | 2.674 | 0.219–32.650 | 0.44 n.s. |
| Grading† | 2.114 | 0.197–22.675 | 0.53 n.s. |
| DG + p27kip1‡ | 12.947 | 1.231–46.090 | 0.032 |
*Risk ratio given as pT2–4 vs pT1 tumors; †risk ratio given as G3–4 vs G1–2 tumors; ‡risk ratio is given as dystroglycan (DG) and p27kip1 low expressor tumors vs the remaining ones. n.s., not significant, RCC, renal cell carcinoma.
To better evaluate the potential cooperative effect of the two markers, four groups of patients were identified: (i) low p27/low DG; (ii) low p27/high DG; (iii) high p27/low DG; and (iv) high p27/high DG and were included in the analysis. Kaplan–Meier curves confirmed the prognostic additive value of the combined analysis showing a significant separation between the four categories of patients both in term of DFS (P = 0.009) and OS (P = 0.008) (Fig. 2c,d). As expected, when the subgroup of low p27/low DG tumors were analyzed separately vs all the remaining cases, the Kaplan–Meier curves displayed a very highly significant separation both in terms of DFS and OS (P < 0.0001) (Fig. 2e,f).
Western blot analysis of p27kip1 and dystroglycan expression levels in renal cell carcinomas. The results obtained by immunohistochemistry indicated that both p27kip1 and α‐DG are differentially expressed in tumor vs normal renal tissues. To get further insights on the mechanisms responsible for these findings, the expression levels of both parameters were analyzed by western blot analyses in a series of 34 RCCs and paired adjacent normal renal tissues (Table 1b). Both α‐ and β‐DG subunits of dystroglycan complex were evaluated and, as shown by representative tumor/normal‐matched tissues in Figure 3(a) (upper panels), there was a clear reduction for the α subunit according to previous data on other human cancers.( 19 , 20 ) In fact, α‐DG levels were reduced in the majority of tumor samples compared to paired normal samples while less obvious were the variations in the expression levels of β‐DG (Fig. 3a, lower panels). Expression levels of p27kip1 varied amongst both normal and tumor samples (Fig. 3b). Surprisingly, western blot analyses did not display a clear reduction in total p27kip1 protein levels between normal vs tumor samples: in fact, p27kip1 levels were decreased, unchanged, or increased in about the same percentage of cases compared to matched normal tissues. The sub‐cellular localization of p27kip1 has been reported to regulate its function in cancer cell proliferation and cytoplasmic redistribution of p27kip1 has been reported in several cancers.( 16 , 21 ) Since we observed a cytoplasmic staining for p27kip1 by immunostaining which was mostly evident in cancer tissues, it was of interest to analyze the subcellular distribution of p27kip1 in RCCs compared to normal tissues. To this aim, nuclear and cytoplasmic protein fractions were prepared from four cases of normal, non‐involved renal tissues, four cases of well‐differentiated (G1–2), and four cases of less‐differentiated (G3–4) RCCs. As shown in Figure 3(c), while in normal tissues p27kip1 displayed a prevalent, if not exclusive, nuclear localization, most of the tumor tissues displayed an evident, and in two cases predominant, cytoplasmic distribution of the protein.
Figure 3.
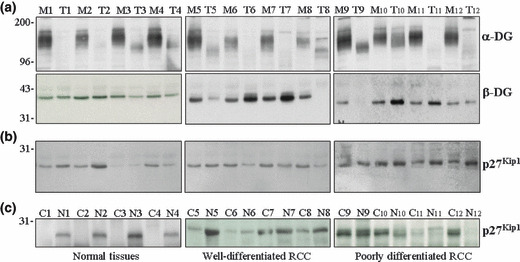
Expression of the dystroglycan (DG) complex and p27kip1 in representative cases of renal cell carcinomas (RCCs). (a) Expression of α‐ and β‐DG subunits in representative cases of RCC (T) and paired normal adjacent tissues (M). (b) Expression of total p27kip1 in the same cases as in (a). (c) Expression of cyctoplasmic (C) and nuclear (N) p27kip1 in representative samples of normal renal tissues and RCCs.
Discussion
In this study, the expression of the CDK inhibitor p27Kip1 and the extent of oxidative DNA damage were evaluated in RCCs and their potential prognostic significance was investigated. In our series of RCCs, we observed a clear reduction of nuclear p27Kip1 expression in cancer tissues which became more evident with increasing tumor grade (Fig. 1 and Table 2). These findings are in agreement with the tumor‐suppressor function of the protein and with previous observations in several human malignancies, including RCC.( 22 , 23 ) p27Kip1 mainly acts in the nucleus and its functional inactivation in tumor cells has been attributed to a proteasome‐dependent degradation of the protein and/or to the cytoplasmic dislocation of the protein.( 16 , 22 ) As shown in Figure 3(c), we found that while in the normal tissue p27Kip1 was mostly (if not exclusively) expressed in the nucleus, in the majority of cancer tissues an evident cytoplasmic band was also observed which in some cases was stronger that the nuclear one. The cytoplasmic delocalization of p27Kip1 in renal cancer cells was recently reported by Kim et al. ( 23 ) who related this phenomenon to activation of AKT and AKT‐dependent phosphorylation of p27Kip1 at T157. These findings are of interest since they might be linked to available evidence suggesting that p27Kip1, in addition to tumor suppressor activities, might also exert oncogenic properties.( 22 , 24 ) In fact, although it is well known that p27−/−mice display an increased susceptibility to tumor formation.( 5 , 22 ) mice with a p27Kip1 variant that does not interact with cyclin/CDK complexes and does not interfere with cell cycle progression develop multiple tumors at a frequency higher than seen with p27+/− mice.( 22 , 24 , 25 ) Thus, it has been suggested that some function(s) of p27Kip1 can enhance tumorigenesis and since they do not refer to its ability to regulate cyclin/CDK complexes, cytoplasmic localization likely plays a role in this phenomenon. Loss of a functional nuclear p27Kip1 protein remains, however, the most important determinant of p27Kip1 involvement in human tumorigenesis.( 22 , 24 ) Indeed, loss of nuclear p27Kip1 has been linked with renal tumorigenesis and has been shown to be associated with increasing tumor grade and to be a risk factor for disease recurrence and cancer‐related death in RCC patients.( 7 , 26 , 27 ) Regardless of the underlying molecular mechanisms, our findings confirmed that loss of nuclear p27Kip1 is a frequent event in RCC and is related to tumor grade as well as to the clinical outcome of patients both in terms of DFS and OS (Fig. 2a,b).
We previously reported that loss of the α subunit of the DG complex, a non‐integrin adhesion molecule, is a frequent event in human renal cancers, correlates with higher tumor grade, and is an independent predictor of early recurrence and death for the disease in clear cell RCC patients.( 4 ) In this study, we further analyzed the DG involvement in renal tumorigenesis and confirmed, by western blot analysis, that the α subunit of the DG complex is frequently reduced in tumor tissues compared to normal adjacent normal tissues, while the β subunit did not display significant variations between normal and tumor tissues. Since DG subunits are encoded by a single gene and are formed upon cleavage of a precursor protein,( 19 , 28 ) detection of the β‐DG subunit in most of the cancers in which a‐DG was not detectable (Fig. 2a) suggests that, as previously reported in other types of human malignancies, this lack of detection is likely not due to loss of gene expression but to a specific post‐translational mechanism affecting α‐DG processing in renal cancer cells.( 4 ) The DG complex connects the ECM network to the cytoskeleton and is likely involved in the regulation of signaling pathways.( 29 ) Thus, regardless of the underlying molecular mechanisms, loss of a functional α‐DG subunit can play an important role in the tumorigenesis process by compromising the formation of strong contacts between ECM and the cytoskeleton of cells resulting, as for integrins, in less sticky tumor cells being able to move unhindered in the ECM, thus predisposed to invade surrounding tissue and metastasize.( 30 ) It will be of interest to evaluate DG expression in the entire process of human renal tumorigenesis (i.e. from early to metastatic lesions).
Since both p27Kip1 and DG( 4 ) exhibited prognostic significance and did not correlate with each other (Table 2), it was of interest to verify whether their combined analysis could add prognostic information for clear cell RCC patients. To this aim, patients were categorized according to p27Kip1 and DG expression. As shown in Figure 2(c,d), the simultaneous loss of both p27kip1 and α‐DG defined a “high‐risk” group of patients who displayed a significantly increased risk of recurrence and death, as also confirmed by multivariate analyses (5, 6).
Another interesting finding of this study is that we were not able to detect an evident oxidative damage, as assessed by evaluating the levels of 8‐OHdG, in RCC tissues compared to normal tissues. Oxidative stress plays an important role in human tumorigenesis and a higher level of oxidative DNA damage has been documented in a variety of human cancers, so it was of interest to analyze it in RCCs. The absence of increased 8‐OHdG levels in more than 75% of cancers suggests that oxidative DNA damage might not be relevant in renal tumorigenesis in which lipid peroxidation, on the other hand, has been reported to play an important role.( 31 ) This finding is in agreement with available data in the literature which show no evidence for a protective effect of any antioxidant nutrient on RCC risk.( 32 ) However, since fully developed malignant lesions were analyzed, this finding does not allow the exclusion of the involvement of oxidative DNA damage in renal tumorigenesis since it might well play a role in earlier steps of the process. Thus, evaluation of oxidative DNA damage in the entire process of human RCC development (i.e. from early to metastatic lesions) is warranted to definitively assess the role of oxidative DNA damage and oxidative stress in renal tumorigenesis.
In conclusion, we investigated p27Kip1 expression in RCCs and demonstrated an inverse relationship with tumor grade. Loss of nuclear p27Kip1 was also shown to be associated with cytoplasmic dislocation of the protein with an increased risk of recurrence and death for disease in patients with clear‐cell RCC.( 1 , 33 ) We previously reported that loss of DG expression is also an independent prognostic marker for clear cell RCC patients. In this study we confirmed this finding in a multivariate analysis including also p27Kip1 and demonstrated that the combined loss of both p27Kip1 and DG is able to identify a subgroup of patients at high risk of recurrence and death. These findings will certainly be of interest, considering the need for useful prognostic indicators able to accurately predict the clinical outcome of patients with localized RCC. However, a prospective multicenter evaluation on a larger population of surgically resected RCCs is warranted to allow a conclusive and definitive assessment of the prognostic significance of p27Kip1 and DG expression level, and to evaluate their suitability in predicting tumor aggressiveness and outcome in RCC patients.
Acknowledgment
This work was supported by grants from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN‐2007N9A3A4) and from Università Cattolica (to A.S.).
References
- 1. Lohse C, Cheville J. A review of prognostic pathologic features and algorithms for patients treated surgically for renal cell carcinoma. Clin Lab Med 2005; 25: 433–64. [DOI] [PubMed] [Google Scholar]
- 2. Sobin L, Wittekind C, (eds). TNM Classification of Malignant Tumors, 6th edn. New York: Wiley, 2002. [Google Scholar]
- 3. Eble J, Sauter G, Epstein J, Sesterhenn Ie. WHO Classification of Tumors. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press, 2004; 65–7. [Google Scholar]
- 4. Sgambato A, Camerini A, Amoroso D et al. Expression of dystroglycan correlates with tumor grade and predicts survival in renal cell carcinoma. Cancer Biol Ther 2007; 6: 1840–6. [DOI] [PubMed] [Google Scholar]
- 5. Sgambato A, Cittadini A, Faraglia B, Weinstein IB. Multiple functions of p27Kip1 and its alterations in tumor cells: a review. J Cell Physiol 2000; 183: 18–27. [DOI] [PubMed] [Google Scholar]
- 6. Langner C, Von Wasielewski R, Ratschek M, Rehak P, Zigeuner R. Biological significance of p27 and Skp2 expression in renal cell carcinoma. A systematic analysis of primary and metastatic tumour tissues using a tissue microarray technique. Wirchows Arch 2004; 445: 631–6. [DOI] [PubMed] [Google Scholar]
- 7. Migita T, Oda Y, Naito S, Tsuneyoshi M. Low expression of p27(Kip1) is associated with tumor size and poor prognosis in patients with renal cell carcinoma. Cancer 2002; 94: 973–9. [PubMed] [Google Scholar]
- 8. Grollman AP, Moriya M. Mutagenesis by 8‐oxoguanine: an enemy within. Trends Genet 1993; 9: 246–9. [DOI] [PubMed] [Google Scholar]
- 9. Yin B, Whyatt RM, Perera FP et al. Determination of 8‐hydroxy‐deoxyguanosine by immunoaffinity chromatography‐monoclonal antibody‐based ELISA. Free Radic Biol Med 1995; 18: 1023–32. [DOI] [PubMed] [Google Scholar]
- 10. Sgambato A, Zannoni G, Faraglia B et al. Decreased expressions of the CDK inhibitor p27 kip 1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol Oncol 2004; 92: 776–83. [DOI] [PubMed] [Google Scholar]
- 11. Fuhrman S, Lasky L, Limas C. Prognostic significance of morfologic parameters in renal carcinoma. Am J Surg Pathol 1982; 6: 655–63. [DOI] [PubMed] [Google Scholar]
- 12. Sgambato A, Migaldi M, Faraglia B et al. Loss of p27Kip1 expression correlates with tumor grade and with reduced disease‐free survival in primary superficial bladder cancers. Cancer Res 1999; 13: 3245–50. [PubMed] [Google Scholar]
- 13. Sgambato A, Migaldi M, Leocata P et al. Loss of p27Kip1 expression is a strong independent prognostic factor of reduced survival in N0 gastric carcinomas. Cancer 2000; 89: 2247–57. [DOI] [PubMed] [Google Scholar]
- 14. Zannoni G, Faraglia B, Tarquini E et al. Expression of the CDK inhibitor p27kip1 and oxidative DNA damage in non‐neoplastic and neoplastic vulvar epithelial lesions. Mod Pathol 2006; 19: 504–13. [DOI] [PubMed] [Google Scholar]
- 15. Sgambato A, De Paola B, Migaldi M et al. Dystroglycan expression is reduced during prostate tumorigenesis and is regulated by androgens in prostate cancer cells. J Cell Physiol 2007; 213: 528–39. [DOI] [PubMed] [Google Scholar]
- 16. Sgambato A, Ratto G, Faraglia B et al. Reduced expression and altered subcellular localization of the CDK inhibitor p27Kip1 in human colon cancer. Mol Carcinog 1999; 26: 172–9. [DOI] [PubMed] [Google Scholar]
- 17. Santella RM. Immunological methods for detection of carcinogen‐DNA damage in humans. Cancer Epidemiol Biomarkers Prev 1997; 216: 166–71. [PubMed] [Google Scholar]
- 18. Yarborouh A, Zhang Y‐J, Hsu T‐M, Santella RM. Immunoperoxidase detection of 8‐hydroxydeoxyguanosine in aflatoxin B1 treated rat liver and human oral mucosa cells. Cancer Res 1996; 56: 683–8. [PubMed] [Google Scholar]
- 19. Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. J Cell Physiol 2005; 205: 163–9. [DOI] [PubMed] [Google Scholar]
- 20. Sgambato A, Migaldi M, Montanari M et al. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am J Pathol 2003; 162: 849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vervoorts J, Luscher J. Post‐translational regulation of the tumor suppressor p27KIP1. Cell Mol Life Sci 2008; 65: 3255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chu I, Hengst L, Slingerland J. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8: 253–67. [DOI] [PubMed] [Google Scholar]
- 23. Kim J, Jonasch E, Alexander A et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin Cancer Res 2009; 15: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sicinski P, Zacharek S, Kim C. Duality of p27Kip1 function in tumorigenesis. Genes Dev 2007; 21: 1703–6. [DOI] [PubMed] [Google Scholar]
- 25. Besson A, Hwang H, Cicero S et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and amultiple tumor phenotype. Genes Dev 2007; 21: 1731–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pertia A, Nikoleishvili D, Trsintsadze O, Gogokhia N, Managadze L, Chkhotua A. Loss of p27(Kip1) CDKI is a predictor of poor recurrence‐free and cancer‐specific survival in patients with renal cancer. Int Urol Nephrol 2007; 39: 381–7. [DOI] [PubMed] [Google Scholar]
- 27. Pertia A, Nikoleishvili D, Trsintsadze O, Gogokhia N, Managadze L, Chkhotua A. Immunoreactivity of p27(Kip1), cyclin D3, and Ki67 in conventional renal cell carcinoma. Int Urol Nephrol 2009; 41: 243–9. [DOI] [PubMed] [Google Scholar]
- 28. Henry MD, Campbell KP. Dystroglycan inside and out. Curr Opin Cell Biol 1999; 11: 602–7. [DOI] [PubMed] [Google Scholar]
- 29. Winder SJ. The complexities of dystroglycan. Trends Biochem Sci 2001; 26: 118–24. [DOI] [PubMed] [Google Scholar]
- 30. Gui GPH, Puddefoot JR, Vinson GP, Wells CA, Carpenter R. Altered cell‐matrix contact: a prerequisite for breast cancer metastasis? Br J Cancer 1997; 75: 623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gago‐Dominguez M, Esteban Castelao J. Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med 2006; 40: 721–33. [DOI] [PubMed] [Google Scholar]
- 32. Bertoia M, Albanes D, Mayne S, Männistö S, Virtamo J, Wright ME. No association between fruit, vegetables, antioxidant nutrients and risk of renal cell carcinoma. Int J Cancer 2010; 126: 1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30. [DOI] [PubMed] [Google Scholar]


