Abstract
We have previously reported that thyroid capsular inflammation induced by sulfadimethoxine (SDM), a goitrogen, might play a role in development of invasive follicular cell adenocarcinomas in rats initiated with N‐bis(2‐hydroxypropyl)nitrosamine (DHPN). The present study was designed to examine the role of cyclooxygenase (COX)‐2, widely known to be up‐regulated in inflammatory states, during chemically induced rat thyroid carcinogenesis. Male F344 rats received a subcutaneous DHPN (2800 mg/kg) injection, and 1 week later were allowed free access to drinking water containing antithyroidal propylthiouracil (PTU, 0.003%) or SDM (0.1%) for 4 or 10 weeks. Control groups receiving goitrogen alone and no treatment were also included. At week 4, diffuse follicular cell hyperplasia was induced in all PTU‐ and SDM‐treated groups, along with fibrous capsular thickening and capsular thickening with inflammation, respectively. Additionally, multiple focal follicular cell hyperplasias and adenomas were observed in the DHPN + PTU and DHPN + SDM cases. At week 10, adenocarcinomas invasive to the capsule and restricted to the capsular adjacent region, were frequent in the DHPN + SDM group, but not observed in the animals given DHPN + PTU. Western blots and immunohistochemistry revealed constitutive COX‐2 expression in non‐neoplastic follicular cells of the control and all of the PTU‐ and SDM‐treated rats. However, COX‐2 reactivity was significantly reduced or negative in the preneoplastic/neoplastic lesions in the DHPN‐treated groups. In fibrous or inflamed thickened capsules, only a few component cells with inflammatory elements were positive for COX‐2, and there was no significant difference in this regard between the PTU and SDM treatments. The present results suggest that capsular inflammation could play a role in development of invasive carcinomas, but COX‐2 expression does not make a major contribution. (Cancer Sci 2005; 96: 31–38)
Continuous treatment with agents inhibiting thyroid peroxidase (TPO), such as sulfonamides, thionamides including 6‐propyl‐2‐thiouracil (PTU), and mercaptomethylimidazole (methimazole), is well known to induce diffuse follicular cell hyperplasia, so‐called goiter, and follicular cell tumors in the long term, as a result of continuous elevation of serum thyroid stimulating hormone (TSH) in rats. 1 , 2 We previously reported rapid induction of invasive follicular cell carcinomas in thickened thyroid capsules with inflammation in a rat 2‐stage carcinogenesis model, which occurred with a sulfonamide, sulfadimethoxine (SDM) treatment after single injection of the genotoxic thyroid carcinogen N‐bis(2‐hydroxypropyl)nitrosamine (DHPN). 1 , 3 We therefore suggested that capsular inflammatory lesions play an important role in the development of invasive thyroid carcinomas. 3 PTU, a thionamide, has also been reported to induce adenomas, but not invasive carcinomas, in rats after DHPN‐initiation, 4 and in this case capsular changes are relatively limited.
Cyclooxygenases (COX), also known as prostaglandin endoperoxidase H synthases, are responsible for two distinct enzymatic activities; that is, as fatty acid cyclooxygenases catalyzing metabolism of arachidonic acid to prostaglandin (PG) G2 and as PG hydroperoxidases converting PGG2 to PGH2. 5 In humans and experimental animals, at least two COX forms, COX‐1 and COX‐2, have been isolated. 6 , 7 , 8 , 9 COX‐1 is recognized as a constitutively, ubiquitously expressed form in most organs and tissues, while COX‐2 is a mitogen‐inducible form, up‐regulated in inflammatory states and cancers. (10) Epidemiological studies have revealed that non‐steroidal anti‐inflammatory drugs with both COX‐1 and COX‐2 inhibitory effects can reduce the incidence of colorectal carcinomas. (11) Furthermore, in animal models, overexpression of COX‐2 has proven to be a potent cause of tumor development, and inhibition of the COX‐2 pathway results in reduction in tumor incidence and progression in the intestines. (12)
The purpose of the present study was to comparatively investigate the effects of goitrogenic TPO‐inhibitors, PTU and SDM, with regard to capsular inflammatory lesions and the development of invasive adenocarcinomas. Immunohistochemistry was applied to determine the distribution/localization of COX‐2, a potent effector molecule in inflammatory states, in inflammatory/neoplastic lesions of rats initiated with DHPN, and evaluate how changes might contribute to carcinogenesis.
Materials and methods
Animal treatment. A total of 84 male Fischer rats (Charles River Japan, Kanagawa, Japan) at 6‐week‐olds were allowed access to a basal diet (CRF‐1, Oriental Yeast, Tokyo, Japan) and water ad libitum, and were kept in polycarbonate cages, with white wood chips for bedding, in an air conditioned room (24 ± 1°C, 55 ± 5% relative humidity; 12 h light and dark cycle). They were divided into five groups, and animals of groups 1 and 3 were initiated with a single subcutaneous injection of DHPN (Nacalai Tesque, Kyoto, Japan) at a dose of 2800 mg/kg bodyweight. Group 2 and 4 animals received vehicle saline only and group 5 was maintained without treatment for normal thyroid and kidney sample collection. One week after the initiation, drinking water containing 0.003% PTU (Sigma Chemical, St. Louis, MO, USA) and 0.1% SDM (Sigma) was provided ad libitum to groups 1 and 2 and groups 3 and 4, respectively, for up to 10 weeks. At weeks 4 and 10 from commencement of the PTU‐ and SDM‐treatment, nine or 10 rats from each group were killed under ether anesthesia for collection of thyroid samples. Controls without any treatment were killed at week 4. The bilateral thyroid lobes were excised, weighed and cut in half. One half of each thyroid lobe from all groups and kidney tissue from normal rats were frozen in liquid nitrogen and preserved at −80°C until use. The other half of each thyroid was fixed in phosphate‐buffered 10% formalin and routinely embedded in paraffin. The experiments were carried out in accordance with the Guide for Animal Experimentation in the National Institute of Health Sciences of Japan.
Histopathology and immunohistochemistry. Serial paraffin‐embedded tissue sections of the largest cut surface of each thyroid lobe were prepared for staining with hematoxylin–eosin and immunohistochemistry. For histopathological evaluation, proliferative lesions of follicular epithelial cells were classified in hematoxylin–eosin stained sections as diffuse hyperplasia, focal hyperplasias, adenomas and adenocarcinomas, according to published criteria. (13) For distinguishing adenocarcinomas from focal hyperplasias/adenomas and other non‐neoplastic lesions, including migrating follicular cells described in the results section, cellular and structural atypia characterized by large nuclei with a fine chromatin pattern and/or nuclear grooves, and irregular glandular structures that were not recognizable as follicles, were fundamental. For immunohistochemistry, antigen retrieval was performed in an autoclave for 10 min at 121°C in 10 mM citrate buffer (pH 6.0). The antirat COX‐2 monoclonal antibody (Transduction Laboratories, Lexington, KY, USA), an anti‐Ki‐67 antigen monoclonal antibody (clone MIB‐5; DAKO, Glostrup, Denmark) for determination of cell proliferative activity, and the streptavidin‐biotin‐peroxidase complex method (StreptABComplex/HRP, DAKO) were used to determine the expression and distribution/localization of each antigen. Sections were lightly counterstained with hematoxylin for microscopic examination. Negative controls without primary antibody reactions were set for each antigen using serial sections. Ki‐67‐positive nuclei per ∼1000 cells of each type of randomly selected follicular lesion were counted.
Western blot analysis. Thyroid samples of four or five rats from each of the experimental groups (1–4) and the normal rat group (5), and kidney samples as a positive control for COX‐2 expression from the normal rats, were homogenized in a commercially available buffer CelLytic MT (Sigma), and supernatants of the lysates were prepared. Equal amounts of protein samples (2.5 µg for thyroid and 1.25–5 µg for kidney) were subjected to SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) on 10% acrylamide gels, and the separated proteins were transferred to polyvinylidene difluoride membranes. Immunoblotting was performed using mouse monoclonal antibodies against the C‐terminal region of the rat COX‐2 protein (clone 33; Transduction Laboratories) or the human β‐actin protein (clone AC‐15; Sigma), followed by exposure to peroxidase‐labeled antimouse IgG goat antibodies (DAKO) and development of signal with the chemiluminescence method (ECL plus Western blotting detection reagents, Amersham Bioscience, Buckinghamshire, UK). Quantification of COX‐2 and β‐actin expression was performed with chemiluminescence detection followed by densitometrical analysis using a DIANA II detection and analysis system (Raytest – Isotopenmessgeraete, Straubenhardt, Germany).
RT‐PCR. RT‐PCR was carried out to confirm constitutive COX‐2 expression in rat organs at the mRNA level. Thyroid and kidney samples from the normal rat group (5) were homogenized in a commercially available reagent, Isogen (Nippon Gene, Toyama, Japan), and total RNA were isolated following the manufacturer's instructions and reverse‐transcribed with oligo(dT) primer and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). This was followed by PCR reactions with Platinum Taq DNA polymerase (Invitrogen) and oligonucleotide primers specific to the 3′‐terminal portion of rat COX‐2 mRNA, including the sequence lacking in COX‐1 mRNA as follows: (9) 5′‐GGATCATCAACACTGCCTCA‐3′ (5′ primer) and 5′‐GCTCAGTTGAACGCCTTTTG‐3′ (3′ primer) (183 bp). Confirmation of PCR‐products was performed with ScaI (Takara Bio, Shiga, Japan), an appropriate restriction enzyme.
Statistics. Statistical analyses to compare the body and thyroid weights, multiplicity of the preneoplastic and neoplastic lesions, Ki‐67 positivity and COX‐2 protein levels were carried out using Student's or Welch's t‐test following F‐test. Incidences of preneoplastic and neoplastic lesions were analyzed with Fisher's exact probability test. Capsular and follicular cell lesions, with grading and the COX‐2 staining pattern, were analyzed using Mann–Whitney's U‐test. Significance was inferred at 5, 1 and 0.1% levels.
Results
Body and thyroid weights. PTU and SDM treatment in groups 1–4, with or without DHPN‐initiation, significantly reduced the final bodyweight as compared to the control group at week 4. The growth inhibition was particularly significant in the PTU‐treated groups at both weeks 4 and 10 (Table 1). Absolute and relative thyroid weights were significantly increased in all experimental groups compared to the control, reflecting goiter and partly tumor development (Table 1). Although the absolute thyroid weights in PTU‐treated groups demonstrated a less significant increase than those in SDM‐treated groups, there was no significant difference in relative thyroid weights between PTU‐ and SDM‐treatment cases (Table 1).
Table 1.
Final body and thyroid weights of goitrogen‐treated rats with or without DHPN initiation
| Group | Treatments | Week | No. animals | Final bodyweight (g) | Absolute thyroid weight (mg) | Relative thyroid weight (mg/100 g bodyweight) |
|---|---|---|---|---|---|---|
| 1 | DHPN + PTU | 4 | 10 | 176.7 ± 8.0 † , ‡ | 97.7 ± 9.9 | 55.2 ± 4.3 |
| 10 | 10 | 170.5 ± 8.7 a , ‡ | 123.8 ± 13.6 ‡ | 72.8 ± 9.0 a , § | ||
| 2 | PTU alone | 4 | 10 | 181.4 ± 7.8 ‡ | 98.3 ± 7.3 ‡ | 54.3 ± 4.8 |
| 10 | 10 | 192.3 ± 9.8 a , ‡ | 115.7 ± 11.0 ¶ | 60.2 ± 5.6 | ||
| 3 | DHPN + SDM | 4 | 9 | 195.8 ± 10.7 | 105.7 ± 11.8 | 53.9 ± 4.8 |
| 10 | 10 | 254.0 ± 13.0 | 162.4 ± 17.5 | 64.0 ± 6.1 | ||
| 4 | SDM alone | 4 | 10 | 205.2 ± 10.2 | 114.1 ± 8.1 | 55.6 ± 2.8 |
| 10 | 10 | 260.1 ± 7.7 | 168.7 ± 22.4 | 64.9 ± 8.2 | ||
| 5 | Control | 4 | 5 | 239.1 ± 5.4 | 10.9 ± 3.0 | 4.6 ± 1.3 |
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; †mean ± SD; § P < 0.05; ‡ P < 0.001 (Student's t‐test); ¶ P < 0.001 (Welch's t‐test) compared with group 3 or 4. Superscript letters indicate significance between values with the same letter: a P < 0.001 (Student's t‐test).
Histopathology. At week 4, diffuse follicular cell hyperplasia, so‐called goiter, was significantly induced in all PTU‐ or SDM‐treated rats of groups 1–4. Additionally, thyroid capsular lesions, which are capsular thickening with or without inflammation, were observed in PTU‐ or SDM‐treated rats. Fibrous thickening of the capsule, with or without minor inflammation, was frequently observed in PTU‐treated rats of groups 1 and 2 (Table 2, Fig. 1A), along with multifocal migration of follicular epithelial cells, forming microfollicles in the thickened capsules (Table 3, Fig. 1A). Severe thickening with inflammation, featuring inflammatory cells and fibroblasts frequently accompanied with diffuse migration of isolated follicular epithelial cells, was observed in the SDM‐treated rats of groups 3 and 4, but not in PTU‐treated rats (2, 3, 1, 2). In addition to the capsular lesions, multiple focal follicular cell hyperplasias and adenomas developed with continuous PTU or SDM treatment after DHPN‐initiation in groups 1 and 3 (Table 4). At week 10, severe fibrous thickening without inflammation remained in the PTU‐treated groups, but inflammation with diffuse follicular cell migration had almost disappeared, with a number of pockets of residual follicular tissue observed in the fibrously thickened capsular layer mainly in the SDM‐treated groups at the 10‐week time point (2, 3). Bilateral follicular necrosis was observed in one of 10 rats receiving SDM alone. Regarding the preneoplastic/neoplastic lesions, focal hyperplasias and adenomas were evident in the DHPN‐treated rats of groups 1 and 3. Adenocarcinomas invading capsules were also detected in seven of 10 rats of DHPN + SDM‐treated group 3, but not in the DHPN + PTU‐treated group 1 (Table 4). All the invasive adenocarcinomas appeared to have arisen in focal hyperplasias/adenomas adjacent to the capsules, and the incipient stage frequently demonstrated evidence of transition from benign neoplasia to malignancy (Fig. 3). No intrathyroidal adenocarcinomas were observed in any of the groups.
Table 2.
Incidences of thyroid capsular lesions in goitrogen‐treated rats, with or without DHPN initiation
| Group | Treatments | Week | No. animals | Fibrous thickening with or without minor inflammation | Thickening with inflammation | ||
|---|---|---|---|---|---|---|---|
| Slight | Severe | Slight | Severe | ||||
| 1 | DHPN + PTU | 4 | 10 | 6 (60) † | 1 (10) a | 3 (30) | 0 c |
| 10 | 10 | 1 (10) | 9 (90) | 0 | 0 | ||
| 2 | PTU alone | 4 | 10 | 5 (50) | 3 (30) b | 2 (20) | 0 d |
| 10 | 10 | 1 (10) | 9 (90) b | 0 | 0 | ||
| 3 | DHPN + SDM | 4 | 9 | 0 | 0 a | 4 (44) | 5 (56) c |
| 10 | 10 | 2 (20) | 8 (80) | 0 | 0 | ||
| 4 | SDM alone | 4 | 10 | 0 | 0 b | 4 (40) | 6 (60) d |
| 10 | 10 | 1 (10) | 9 (90) | 0 | 0 | ||
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; †percentage. Superscript letters indicate significance between values with the same letter: a,b,c P < 0.01, d P < 0.001 (Mann–Whitney's U‐test).
Figure 1.
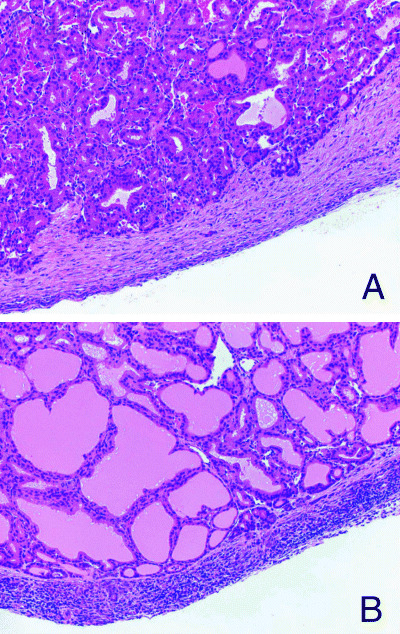
Capsular lesions of rat thyroids. (A) Fibrous thickening with minor inflammatory cell infiltration in a rat treated with 6‐propyl‐2‐thiouracil for 4 weeks. Note focal migration of follicular epithelial cells forming microfollicles in the capsule. HE (×180). (B) Severe thickening with inflammation in a rat treated with sulfadimethoxine for 4 weeks HE (×180).
Table 3.
Incidence of follicular epithelial cell lesions in thyroid capsules of goitrogen‐treated rats with or without DHPN initiation
| Group | Treatments | Week | No. animals | Migration of follicular epithelial cells in capsules | Pockets of residual follicular tissue in capsules | ||
|---|---|---|---|---|---|---|---|
| Multifocal | Diffuse | Number/rat | |||||
| 1–3 | 3< | ||||||
| 1 | DHPN + PTU | 4 | 10 | 6 (60) † | 1 (10) a | 4 (40) | 0 |
| 10 | 10 | 10 (100) | 0 | 7 (70) | 0 [Link] , [Link] | ||
| 2 | PTU alone | 4 | 10 | 9 (90) | 1 (10) b | 6 (60) | 0 |
| 10 | 10 | 9 (90) | 0 | 6 (60) | 4 (40) c | ||
| 3 | DHPN + SDM | 4 | 9 | 3 (33) | 6 (67) a | 2 (22) | 0 |
| 10 | 10 | 10 (100) | 0 | 4 (40) | 6 (60) d | ||
| 4 | SDM alone | 4 | 10 | 3 (30) | 7 (70) b | 1 (10) | 0 |
| 10 | 10 | 10 (100) | 0 | 2 (20) | 8 (80) | ||
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; †percentage. Superscript letters indicate significance between values with the same letter: a,b,c P < 0.05; d P < 0.01 (Mann–Whitney's U‐test).
Figure 2.
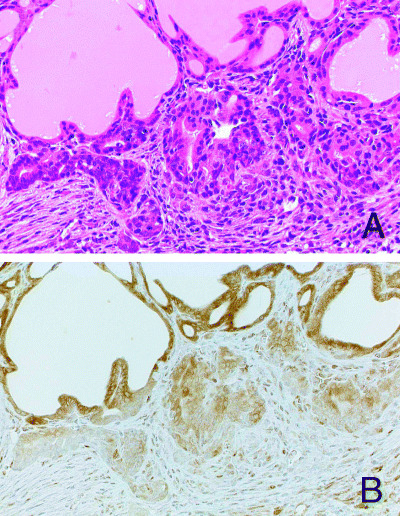
Diffuse migration of isolated follicular epithelial cells into an inflamed capsule in a rat treated with sulfadimethoxine for 4 weeks. (A) HE (×360). (B) Significant reduction of COX‐2 expression in the migrating follicular cells. Only a few interstitial inflammatory cells, fibroblasts and endothelial cells show positive reactivity for COX‐2 antibodies.
Table 4.
Incidences and multiplicities of thyroid preneoplastic and neoplastic lesions in goitrogen‐treated rats after DHPN initiation
| Group | Treatments | Week | No. animals | Focal hyperplasias | Adenomas | Adenocarcinomas invasive to capsules |
|---|---|---|---|---|---|---|
| 1 | DHPN + PTU | 4 | 10 | 10 † (5.1 ± 1.7 ‡ ) | 2 (0.2 ± 0.4) | 0 |
| 10 | 10 | 10 (12.5 ± 2.9) | 9 (7.0 ± 3.1) | 0 | ||
| 3 | DHPN + SDM | 4 | 9 | 9 (7.7 ± 2.7) | 3 (0.3 ± 0.5) | 0 |
| 10 | 10 | 10 (15.6 ± 5.3) | 10 (5.0 ± 2.0) | 7* (1.0 ± 0.9) |
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; †incidence; ‡multiplicity; *P < 0.01 compared with group 2 (Fisher's exact probability test).
Figure 3.
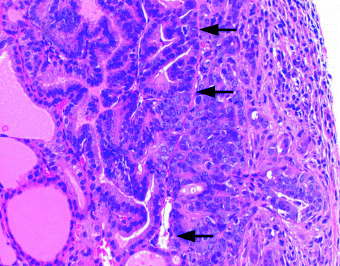
An incipient stage of invasive carcinoma (right) arising from a focal hyperplasia (upper left). Arrows indicate the original capsular line. Transcapsular invasion of atypical carcinoma cells with a large nuclei, with fine chromatin pattern is evident HE (×360).
Immunohistochemistry. In inflamed or fibrous thickened thyroid capsules, only a few component cells including inflammatory cells, fibroblasts and endothelial cells stained positive for COX‐2 in the PTU‐ or SDM‐treated rats (Fig. 2B), and there were no significant differences in the COX‐2 positive cell populations between the two cases. In contrast, cytoplasmic immunoreactivity for COX‐2 was detected in normal follicular cells in the control rats (Fig. 4A) and in the diffusely hyperplastic follicular cells of the PTU‐ or SDM‐treated rats. In contrast, preneoplastic and neoplastic follicular lesions, including focal hyperplasias, adenomas and adenocarcinomas, were frequently negative or only very weakly reactive for COX‐2 in the DHPN‐treated rats (Fig. 4B,C). Data for COX‐2‐immunoreactivity in the lesions are summarized in Table 5. At week 4, 87–94 and 100% of focal hyperplasias and adenomas, respectively, were COX‐2 negative and the values were lower but still 45–82% at week 10, with the more advanced lesions having the highest percentages. Observed in both the PTU‐ and SDM‐treated groups, follicular epithelial cells migrating into capsules at week 4 and pockets of residual follicles in thickened capsules at week 10 were also negative or only weakly reactive for COX‐2 (Fig. 2B).
Figure 4.
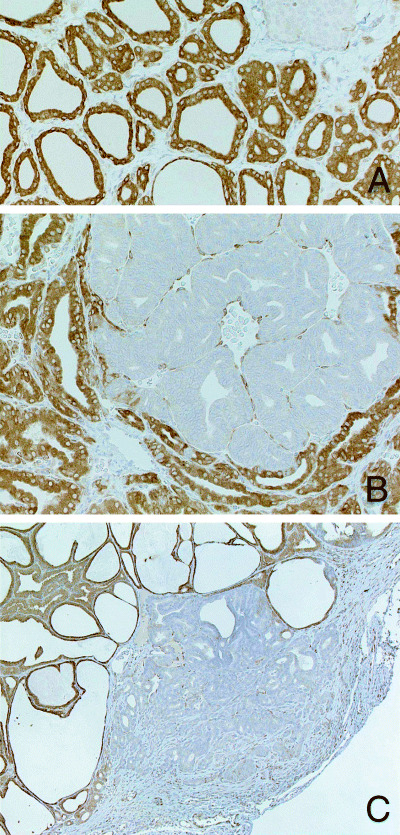
(A) Cytoplasmic positivity for COX‐2 is evident in follicular cells in a control rat. The COX‐2 negative tissue shown on the upper right is parathyroid. Immunohistochemistry (×360). (B) Marked reduction of COX‐2 expression in neoplastic lesions of thyroid follicular cells. Adenoma in a rat treated with PTU for 4 weeks after DHPN‐initiation. Immunohistochemistry (×360). (C) Adenocarcinoma invading into the thyroid capsule in a rat treated with SDM for 10 weeks after DHPN‐initiation. Immunohistochemistry (×140).
Table 5.
COX‐2‐immunoreactivity in thyroid preneoplastic and neoplastic lesions in goitrogen‐treated rats after DHPN initiation
| Group | Treatments | Week | Focal hyperplasias | Adenomas | Adenocarcinomas invasive to capsules | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (–) | (+) | (++) | n | (–) | (+) | (++) | n | (–) | (+) | |||
| 1 | DHPN + PTU | 4 | 51 | 48 (94) † | 3 (6) | 0 (0) b | 2 | 2 (100) | 0 (0) | 0 (0) | 0 | ||
| 10 | 125 | 56 (45) | 55 (44) | 14 (11) b | 70 | 43 (61) | 25 (36) | 2 (3) | 0 | ||||
| 3 | DHPN + SDM | 4 | 69 | 60 (87) | 9 (13) | 0 (0) a | 3 | 3 (100) | 0 (0) | 0 (0) | 0 | ||
| 10 | 156 | 101 (65) | 55 (35) | 0 (0) a | 50 | 41 (82) | 9 (18) | 0 (0) | 10 | 9 (90) | 1 (10) | ||
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; n, number of lesions; †percentage; (–), negative; (+), positive in 20–90% cells; (++), positive in >90% cells. Superscript letters indicate significance between values with the same letter: a P < 0.01; b P < 0.001 (Mann–Whitney's U‐test).
Cell proliferative activity. Immunohistochemistry for Ki‐67 revealed an increase in cell proliferative activity of follicular cells in the preneoplastic and neoplastic focal hyperplasias, adenomas and/or adenocarcinomas, as compared to surrounding follicles, in rats treated with PTU or SDM after DHPN‐initiation (data not shown). Ki‐67 labeling indices in adenomas and surrounding follicles with reference to staining patterns for COX‐2 assessed using serial sections at week 10 are summarized in Table 6. There was no difference between COX‐2‐negative and ‐positive adenomas.
Table 6.
Ki‐67‐positivity in thyroid adenomas with or without COX‐2 expression in goitrogen‐treated rats after DHPN initiation
| Group | Treatments | Week | Adenomas | Adenomas | Surrounding follicles | |||
|---|---|---|---|---|---|---|---|---|
| n | COX‐2 (–) | n | COX‐2 (+/++) | n | COX‐2 (++) | |||
| 1 | DHPN + PTU | 10 | 10 | 9.0 ± 4.3*, † | 10 | 9.1 ± 5.7* | 10 | 1.9 ± 0.3 |
| 3 | DHPN + SDM | 10 | 10 | 10.2 ± 3.1* | 9 | 9.6 ± 3.8* | 10 | 1.9 ± 0.7 |
DHPN, N‐bis(2‐hydroxypropyl)nitrosamine; PTU, 6‐propyl‐2‐thiouracil; SDM, sulfadimethoxine; n, number of lesions; †mean ± SD. *P < 0.01 compared with surrounding follicles (Welch's t‐test).
Western blot analysis and RT‐PCR. Western blot analysis using the anti COX‐2 monoclonal antibody revealed a band at ∼72 kDa in the thyroids of all groups and kidneys of the control rats of group 5 (Fig. 5A), which corresponds with previous studies. 14 , 15 Quantitation by densitometric scanning and normalization for β‐actin levels of the thyroid samples showed significant reduction of COX‐2 expression with or without statistical significance to 55–73% and 35–51% of the control rat level in the PTU‐ and SDM‐treated rats, respectively. In the SDM‐treated rats the levels were much lower than those in PTU‐treated counterparts (Fig. 5B). Constitutive COX‐2 mRNA expression in thyroid and kidney tissues in the no treatment rats of group 5 was confirmed by RT‐PCR using specific primers (Fig. 5C).
Figure 5.
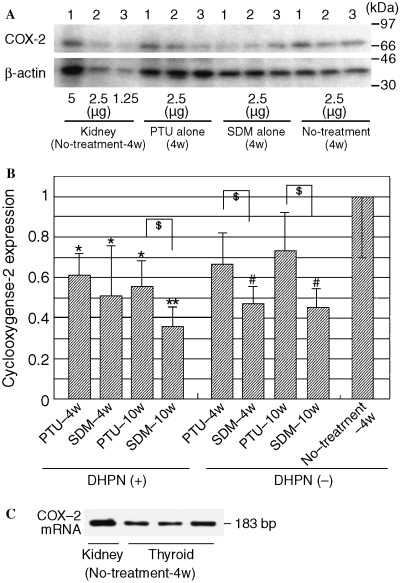
Western blot analysis of COX‐2 from thyroids of PTU or SDM‐treated rats with or without DHPN‐initiation and normal rats without any treatment, and results of RT‐PCR for COX‐2 mRNA in normal rat organs. (A) Analysis using anti‐COX‐2 monoclonal antibody reveals constitutive expression of a band in the thyroids and kidneys of rats, with or without PTU or SDM treatment. (B) Quantitative values of COX‐2 in thyroids, presented as mean ± SD, normalized for β‐actin levels in the same tissue sample. Densities for COX‐2 and β‐actin protein were calculated with analytical curves of 1.25 µg to 5.0 µg control rat kidneys for each blot. *,**P < 0.05, 0.01 compared to no‐treatment‐4w (Student's t‐test); $P < 0.05 compared to no‐treatment‐4w (Welch's t‐test); #P < 0.05 (Student's t‐test). (C) RT‐PCR results using oligonucleotide primers specific to the 3′‐terminal portion of rat COX‐2 mRNA, including the sequence lacking in the COX‐1 mRNA.
Discussion
At week 4 of the present carcinogenesis model, fibrous thickening of the capsule with or without minor inflammation was frequently observed in PTU‐ but not SDM‐treated rats, with or without DHPN‐initiation. In previous studies, thiouracil, an antithyroidal compound related to PTU, was reported to induce severe capsular thickening with inflammation between weeks 2 and 4, followed by their disappearance and capsular fibrous thickening. (16) Therefore, the present PTU‐induced fibrous capsular thickening at week 4 could be considered a consequence of such inflammatory changes. In contrast, thickening with much more extensive inflammation was still evident in the SDM case. In addition to capsular lesions, multiple focal follicular cell hyperplasias and a few small adenomas also developed in the DHPN + PTU and DHPN + SDM group rats. At 10 weeks the inflammatory reactions had largely disappeared, leaving slight to severe fibrous thickening of capsules in the SDM‐treated animals. The numbers and sizes of focal hyperplasias and adenomas had increased in the DHPN + PTU and DHPN + SDM groups, as compared to the earlier time point, with no significant intergoitrogen differences in incidence or multiplicity. However, invasive adenocarcinomas that appeared to have arisen in focal hyperplasias/adenomas adjacent to the capsule, were only observed in the DHPN + SDM‐treated group, and we hypothesize on the basis of these histopathological characteristics that capsular inflammatory change plays a role in tumor progression. In our previous sequential analysis, focal hyperplasias/adenomas were seldom observed at week 2 but were obviously increased at week 4 in DHPN + SDM‐treated rats, (3) and there is a possibility that coincidental and contiguous appearance of inflammatory and preneoplastic/neoplastic lesions in the period around week 4 contributes to acceleration of invasive carcinoma development. In the PTU case, inflammatory and preneoplastic/neoplastic lesions are speculated not to meet these conditions.
Whether there is any inflammatory factor restricted to the capsular region in the present rat model is not clear. Autoimmune thyroiditis induced by thyroglobulin (Tg) or thyroid peroxidase (TPO) is widely known in patients and experimental animals. 17 , 18 Under normal conditions, Tg is synthesized by follicular cells and secreted into the follicular lumen, where it is stored. TPO is known to be localized mainly in apical surfaces, which face the follicular lumen. (19) However, in the present SDM‐treated rats, with and without DHPN‐initiation, diffuse migration of isolated follicular epithelial cells at week 4, which is thought to contribute to the presentation of Tg or TPO to the immune system, was frequently observed along with inflammation. Therefore, Tg or TPO might be suspected as possible inflammatory factors.
COX‐2 is widely known to be an inducible form in most tissues, up‐regulated in inflammatory states as well as in cancers, and it has been recognized to contribute to cell proliferation, angiogenesis or immunologic function in tumors. (20) A number of animal models have revealed COX‐2 inhibitors to significantly suppress intestinal tumor development. 11 , 21 As an original aim of the immunohistochemistry in the present study, distribution/localization of COX‐2 in thyroids with inflammatory/neoplastic lesions were evaluated for their contribution to carcinogenesis, but only a few component cells of the capsular inflammatory region stained positive for COX‐2 in the PTU‐ or SDM‐treated all rats; and there were no significant differences in the COX‐2 positive cell populations between the two cases. In contrast, immunohistochemistry, Western blot analysis, and RT‐PCR revealed constitutive COX‐2/COX‐2 mRNA expression in follicular cells of not only the PTU‐ and SDM‐treated rats, but also the control rats. Constitutive COX‐2 expression has been found in several normal organs, including human and animal kidneys, (22) pancreas, (23) brains, 24 , 25 ovaries, 26 , 27 and human thyroids. (28) Physiological roles of constitutively expressed COX‐2, such as secretory regulation of renin and insulin in kidneys and the endocrine pancreas, respectively, 22 , 29 have been clarified but functions in the thyroid are yet to be detailed. The present study showed for the first time that COX‐2 is constitutively expressed in normal rat thyroid follicular cells, so that they can be used as models for investigating physiological significance. In previous studies, results for COX‐2 expression in normal thyroid tissue of humans have been contradictory. In some cases, immunohistochemistry demonstrated negative or occasional positive staining. 30 , 31 , 32 However, COX‐2 protein and mRNA could be detected using Western and Northern blots and/or immunohistochemistry. 28 , 33 Although the cause of the discrepancy is not clear, COX‐2 expression in normal follicular cells in humans might be relatively low compared to the rat case.
The most striking aspect in the present study was the general lack of expression of COX‐2 in follicular cell preneoplastic and neoplastic lesions in rats treated with PTU or SDM after DHPN‐initiation, and Western blot analysis also revealed significant reduction in COX‐2‐expression in thyroids of these animals. Furthermore, analysis of cell proliferative activity showed no differences between COX‐2‐negative and ‐positive adenomas, both exhibiting a significant increase in labeling compared to the surrounding follicular tissues. In human thyroid tumors, contradictory results have been documented, with over‐expression of COX‐2 found in papillary carcinomas, 32 , 33 whereas expression was found to be less frequent or lacking in follicular carcinomas and/or undifferentiated carcinomas. 30 , 32 Others have demonstrated up‐regulation in malignant follicular cell tumors but not in benign lesions. (31) Overall it can be concluded that the follicular cells in preneoplastic/neoplastic lesions of the present rat thyroid carcinogenesis model, and some advanced human thyroid carcinomas, do not require COX‐2 expression for their high growth activities. A novel mechanism of overexpression of COX‐2 with inhibition of cell proliferation has been proposed on the basis of research with a variety of cells in culture, (34) and a restricted subpopulation of cells in the rat kidney, and that is independent of PG synthesis but involves p53, p21cip−1, and p27kip−1. (35) The present results indicate that modulatory effects of COX‐2 are disrupted or follicular cells themselves are dedifferentiated during carcinogenesis, resulting in a significant reduction of expression and enhanced cell proliferative activity in preneoplastic and neoplastic lesions in the rat thyroid. Investigation of interactions between COX‐2 and expression of tumor suppressor proteins or cyclin‐dependent kinase inhibitors and underlying regulatory mechanisms is clearly warranted using the present rat model.
Follicular epithelial cells migrating into capsules at week 4 and pockets of residual follicles in thickened capsules at week 10, which were observed in both PTU‐ and SDM‐treated groups with and without DHPN‐initiation, also proved negative or only weakly positive for COX‐2. In thyroiditis patients, increased expression in follicular cells has been reported, perhaps reflecting the fundamental nature of COX‐2 as an inducible form. 30 , 33 Therefore, the cause of its down‐regulation in migrating cells in the present rat study might not be correlated to capsular inflammation, and one possibility might be decreased intercellular communication resulting in dedifferentiation.
In summary, continuous SDM, but not PTU treatment, induced severe capsular inflammation followed by frequent invasive follicular cell carcinomas in association with thickened capsules in the present rat thyroid carcinogenesis model. Only a few inflammatory cells, fibroblasts and endothelial cells in the capsules were positive for COX‐2 in the PTU‐ or SDM‐treated rats, and significant reduction of COX‐2 expression was observed in preneoplastic/neoplastic lesions in rats treated with PTU or SDM after DHPN‐initiation. These results suggest that capsular inflammation might play a role in the development of invasive adenocarcinomas in rats treated with SDM after DHPN‐initiation, but that COX‐2 expression is not a major contributor in this process.
Acknowledgment
This study was supported by a Grant‐in‐Aid from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Mitsumori K, Onodera H, Takahashi M, Shimo T, Yasuhara K, Kitaura K, Hayashi Y. Effect of thyroid stimulating hormone on the development and progression of rat thyroid follicular cell tumors. Cancer Lett 1995; 92: 193–202. [DOI] [PubMed] [Google Scholar]
- 2. Hood A, Liu YP, Gattone VH. 2nd, Klaassen CD. Sensitivity of thyroid gland growth to thyroid stimulating hormone (TSH) in rats treated with antithyroid drugs. Toxicol Sci 1999; 49: 263–71. [DOI] [PubMed] [Google Scholar]
- 3. Imai T, Onose J, Hasumura M, Ueda M, Takizawa T, Hirose M. Sequential analysis of development of invasive thyroid follicular cell carcinomas in inflamed capsular regions of rats treated with sulfadimethoxine after N‐bis (2‐hydroxypropyl) nitrosamine‐initiation. Toxicol Pathol 2004; 32: 229–36. [DOI] [PubMed] [Google Scholar]
- 4. Hiasa Y, Kitahori Y, Kato Y, Ohshima M, Konishi N, Shimoyama T, Sakaguchi Y, Hashimoto H, Minami S, Murata Y. Potassium perchlorate, potassium iodide, and propylthiouracil: promoting effect on the development of thyroid tumors in rats treated with N‐bis (2‐hydroxypropyl) nitrosamine. Jpn J Cancer Res 1987; 78: 1335–40. [PubMed] [Google Scholar]
- 5. Ohki S, Ogino N, Yamamoto S, Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem 1979; 254: 829–36. [PubMed] [Google Scholar]
- 6. Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun 1989; 165: 888–94. [DOI] [PubMed] [Google Scholar]
- 7. Hla T, Neilson K. Human cyclooxygenase‐2 cDNA. Proc Natl Acad Sci USA 1992; 89: 7384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy BP, Chan CC, Culp SA, Cromlish WA. Cloning and expression of rat prostaglandin endoperoxide synthase (cyclooxygenase)‐2 cDNA. Biochem Biophys Res Commun 1993; 197: 494–500. [DOI] [PubMed] [Google Scholar]
- 9. Feng L, Sun W, Xia Y, Tang WW, Chanmugam P, Soyoola E, Wilson CB, Hwang D. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch Biochem Biophys 1993; 307: 361–8. [DOI] [PubMed] [Google Scholar]
- 10. Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)‐1 and ‐2. J Biol Chem 1996; 271: 33157–60. [DOI] [PubMed] [Google Scholar]
- 11. Taketo MM. Cyclooxygenase‐2 inhibitors in tumorigenesis (Part II). J Natl Cancer Inst 1998; 90: 1609–20. [DOI] [PubMed] [Google Scholar]
- 12. Cao Y, Prescott SM. Many actions of cyclooxygenase‐2 in cellular dynamics and in cancer. J Cell Physiol 2002; 190: 279–86. [DOI] [PubMed] [Google Scholar]
- 13. Hardisty JF, Boorman GA. Thyroid gland. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, MacKenzie WF (eds). Pathology of the Fischer Rat. Academic Press, San Diego 1990; 519–36. [Google Scholar]
- 14. Gianoukakis AG, Cao HJ, Jennings TA, Smith TJ. Prostaglandin endoperoxide H synthase expression in human thyroid epithelial cells. Am J Physiol Cell Physiol 2001; 280: C701–8. [DOI] [PubMed] [Google Scholar]
- 15. Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res 2001; 61: 1927–33. [PubMed] [Google Scholar]
- 16. Wollman SH, Herveg JP. Thyroid capsule changes during the development of thyroid hyperplasia in the rat. Am J Pathol 1978; 93: 639–54. [PMC free article] [PubMed] [Google Scholar]
- 17. Ng HP, Banga JP, Kung AW. Development of a murine model of autoimmune thyroiditis induced with homologous mouse thyroid peroxidase. Endocrinology 2004; 145: 809–16. [DOI] [PubMed] [Google Scholar]
- 18. Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: what can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology 2004; 112: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuliawat R, Lisanti MP, Arvan P. Polarized distribution and delivery of plasma membrane proteins in thyroid follicular epithelial cells. J Biol Chem 1995; 270: 2478–82. [DOI] [PubMed] [Google Scholar]
- 20. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999; 18: 7908–16. [DOI] [PubMed] [Google Scholar]
- 21. Fukutake M, Nakatsugi S, Isoi T, Takahashi M, Ohta T, Mamiya S, Taniguchi Y, Sato H, Fukuda K, Sugimura T, Wakabayashi K. Suppressive effects of nimesulide, a selective inhibitor of cyclooxygenase‐2, on azoxymethane‐induced colon carcinogenesis in mice. Carcinogenesis 1998; 19: 1939–42. [DOI] [PubMed] [Google Scholar]
- 22. Harris RC, Breyer MD. Physiological regulation of cyclooxygenase‐2 in the kidney. Am J Physiol Renal Physiol 2001; 281: F1–11. [DOI] [PubMed] [Google Scholar]
- 23. Sorli CH, Zhang HJ, Armstrong MB, Rajotte RV, Maclouf J, Robertson RP. Basal expression of cyclooxygenase‐2 and nuclear factor‐interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci USA 1998; 95: 1788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX‐2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA 1996; 93: 2317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bazan NG, Lukiw WJ. Cyclooxygenase‐2 and presenilin‐1 gene expression induced by interleukin‐1beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J Biol Chem 2002; 277: 30359–67. [DOI] [PubMed] [Google Scholar]
- 26. Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis‐ acting C/EBP beta promoter element. J Biol Chem 1993; 268: 21931–8. [PubMed] [Google Scholar]
- 27. Narko K, Ritvos O, Ristimaki A. Induction of cyclooxygenase‐2 and prostaglandin F2alpha receptor expression by interleukin‐1beta in cultured human granulosa‐luteal cells. Endocrinology 1997; 138: 3638–44. [DOI] [PubMed] [Google Scholar]
- 28. Smith TJ, Jennings TA, Sciaky D, Cao HJ. Prostaglandin‐endoperoxide H synthase‐2 expression in human thyroid epithelium. Evidence for constitutive expression in vivo and in cultured KAT‐50 cells. J Biol Chem 1999; 274: 15622–32. [DOI] [PubMed] [Google Scholar]
- 29. Robertson RP. Dominance of cyclooxygenase‐2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes 1998; 47: 1379–83. [DOI] [PubMed] [Google Scholar]
- 30. Cornetta AJ, Russell JP, Cunnane M, Keane WM, Rothstein JL. Cyclooxygenase‐2 expression in human thyroid carcinoma and Hashimoto's thyroiditis. Laryngoscope 2002; 112: 238–42. [DOI] [PubMed] [Google Scholar]
- 31. Specht MC, Tucker ON, Hocever M, Gonzalez D, Teng L, Fahey TJ, III. Cyclooxygenase‐2 expression in thyroid nodules. J Clin Endocrinol Metab 2002; 87: 358–63. [DOI] [PubMed] [Google Scholar]
- 32. Ito Y, Yoshida H, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Matsuura N, Kuma K, Miyauchi A. Cyclooxygenase‐2 expression in thyroid neoplasms. Histopathology 2003; 42: 492–7. [DOI] [PubMed] [Google Scholar]
- 33. Nose F, Ichikawa T, Fujiwara M, Okayasu I. Up‐regulation of cyclooxygenase‐2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 2002; 117: 546–51. [DOI] [PubMed] [Google Scholar]
- 34. Trifan OC, Smith RM, Thompson BD, Hla T. Overexpression of cyclooxygenase‐2 induces cell cycle arrest. Evidence for a prostaglandin‐independent mechanism. J Biol Chem 1999; 274: 34141–7. [DOI] [PubMed] [Google Scholar]
- 35. Zahner G, Wolf G, Ayoub M, Reinking R, Panzer U, Shankland SJ, Stahl RA. Cyclooxygenase‐2 overexpression inhibits platelet‐derived growth factor‐induced mesangial cell proliferation through induction of the tumor suppressor gene p53 and the cyclin‐dependent kinase inhibitors p21waf‐1/cip‐1 and p27 kip‐1. J Biol Chem 2002; 277: 9763–71. [DOI] [PubMed] [Google Scholar]


