Figure 4.
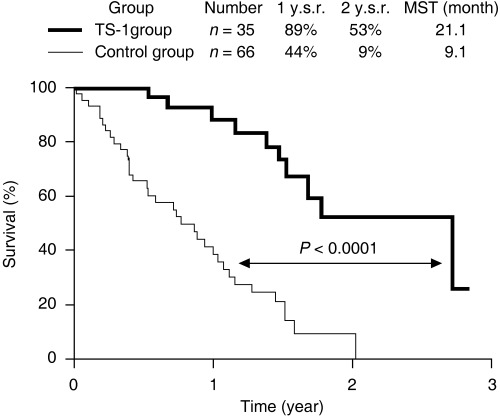
Survival improvement by TS‐1 in patients with peritoneal free cancer cells without macroscopic peritoneal dissemination (P0/Cy1 status). After radical gastrectomy, 35 patients were treated with oral TS‐1 (80 mg/m2) for 28 consecutive days with 14 days rest, and the schedule was repeated every 6 weeks (TS‐1 group). The other 66 patients did not receive any chemotherapy (control group). Patients in the TS‐1 group survived significantly longer than those in the control group (P < 0.0001). The Cox proportional hazard model showed TS‐1 treatment as an independent prognostic factor, and the relative risk for TS‐1 treatment was 0.17‐fold lower than that for the control group. Major adverse reactions included myelosuppression and gastrointestinal toxicities, but they were generally mild and there were no treatment‐related deaths. 1 y.s.r and 2 y.s.r., 1‐year and 2‐year survival rates; MST, median survival time.
