Figure 1.
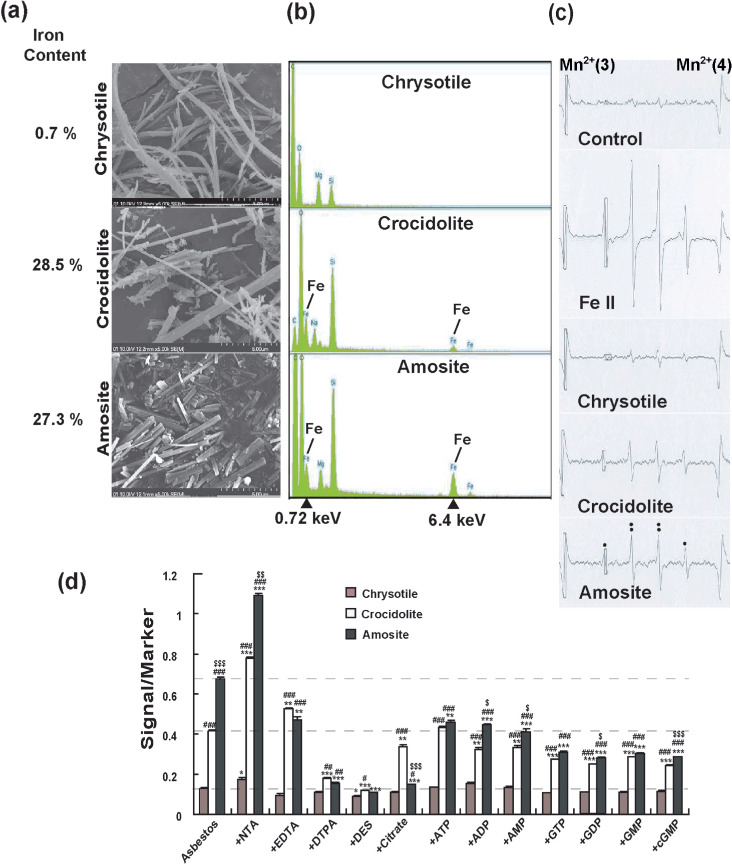
Physicochemical features of asbestos and its modification by iron chelators. (a) Scanning electron microscopy images of chrysotile, crocidolite and amosite with different iron content (×5000). Each asbestos presented a different appearance. Iron content is from a reference paper.( 26 ) (b) Energy dispersive X‐ray spectroscopic spectrum of chrysotile, crocidolite and amosite. Crocidolite and amosite showed high content of iron. (c) Electron spin resonance (ESR) analyses of chrysotile, crocidolite and amosite in the presence of H2O2. Ferrous iron was used as positive control. The signals are those of hydroxyl radicals. (d) ESR analyses of chrysotile, crocidolite and amosite in the presence of H2O2 and different iron chelators. n = 3, means ± standard error of the mean; *P < 0.05, **P < 0.01, ***P < 0.001 vs each asbestos plus H2O2 alone; # P < 0.05, ## P < 0.01, ### P < 0.001 vs chrysotile plus H2O2 within each chelator group; $ P < 0.05, $$ P < 0.01, $$$ P < 0.001 vs crocidolite plus H2O2 within each chelator group. ADP, adenosine 5′‐disphosphate; AMP, adenosine 5′‐monophosphate; ATP, adenosine 5′‐triphosphate; cGMP, cyclic guanosine monophosphate; DES, desferal; DTPA, diethylenetriaminepentaacetic acid; EDTA, ethylenediaminetetraacetic acid; GDP, guanosine 5′‐diphosphate; GMP, guanosine 5′‐monophosphate; GTP, guanosine 5′‐triphosphate; NTA, nitrilotriacetic acid.
