Figure 3.
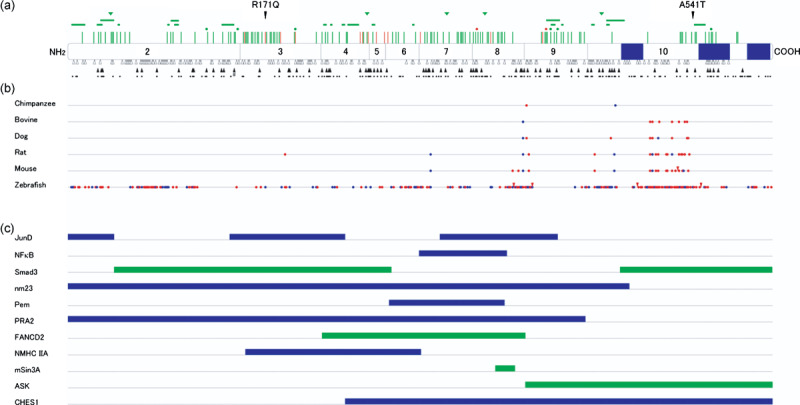
Mutations, evolutionary diversion, and binding domains of mein. (a) Germline MEN1 mutations identified in multiple endocrine neoplasia type 1 (MEN1) and related disorders. Locations of missense mutations (perpendicular lines), in‐frame deletions (dot, one amino acid; horizontal bar, two or more amino acids), and in‐frame insertions (triangles) are shown above the diagram of menin, with corresponding exons numbered. Green symbols indicate mutations causing MEN1, and orange symbols indicate those causing familial isolated hyperparathyroidism or apparently sporadic parathyroid tumor. Frameshift mutations (open triangles) and nonsense mutations (closed triangles) are shown below menin, with potential nonsense mutation sites indicated by dots. Splicing mutations and large deletions are not depicted. Blue boxes indicate nuclear localization signals. The normal polymorphisms R171Q and A541T are also indicated. #Nonsense mutation involving two base pair substitutions. (b) Homology between menin amino acid sequences among different vertebrate species. Sequences were retrieved from the internet website: http://www.ncbi.nlm.nih.gov/HomoloGene/. Blue and red dots indicate conservative and non‐conservative amino acid replacement compared with human menin, respectively. Red triangles indicate insertions of amino acid residues. (c) Menin regions implicated in its binding to interacting proteins. Blue bars represent the regions required for binding to proteins demonstrated to bind directly to menin. Green bars indicate the regions required for association with proteins not shown to bind directly to menin.
