Figure 1.
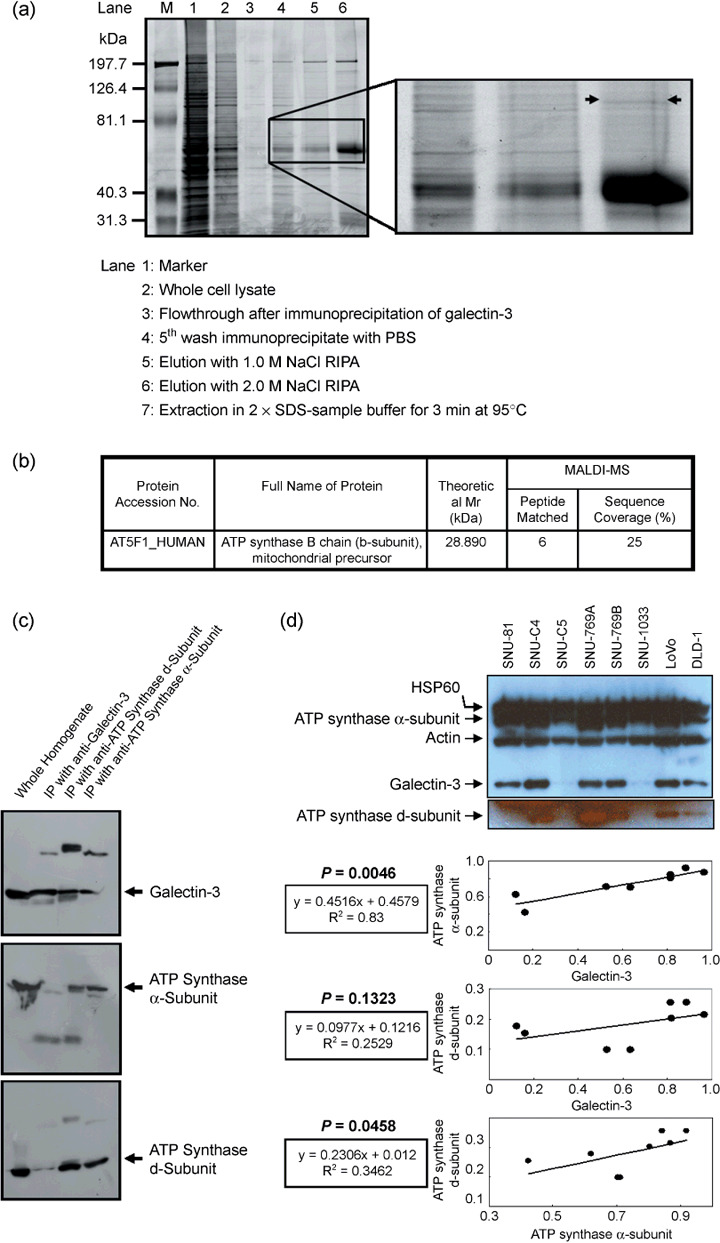
Identification of mitochondrial F1F0‐ATP synthase (ATP synthase) b‐subunit in the antigalectin‐3 antibody immunoprecipitate. (a) Sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE) of the galectin‐3 antibody immunoprecipitate. The galectin‐3 immunoprecipitate was fractionated using a salt gradient, as shown in the figure, and subjected to SDS‐PAGE. (b) Identification of the ATP synthase b‐subunit in the immunoprecipitate by matrix‐assisted laser desorption ionization–time of flight analysis. After staining the SDS‐PAGE gel, the protein band specified in (a) was excised and in‐gel digested to determine the peptide mass. The protein was successfully identified as the ATP synthase b‐subunit, a mitochondrial precursor. (c) Interactions of the ATP synthase d‐subunit with galectin‐3. Immunoprecipitates of the antigalectin‐3 antibody were probed with commercially available anti‐ATP synthase α‐ or d‐subunit antibodies, and vice‐versa. Immunoreactive signals of the ATP synthase d‐subunit were clearly detected in immunoprecipitates of antigalectin‐3 and anti‐ATP synthase α‐subunit antibodies. (d) Positive correlation of galectin‐3 expression with ATP synthase α‐ and d‐subunits in eight individual human colon cancer cell lines. Expression of galectin‐3 and ATP synthase subunits was normalized with actin and heat shock protein (HSP) 60, respectively. OD, arbitrary unit.
