Abstract
Background:
Elevated platelet lymphocyte ratio (PLR) and low body mass index (BMI) are associated with inferior survival in non-small cell lung cancer (NSCLC) patients receiving immunotherapy (IO). We evaluated real-world prognostic utility of PLR, BMI, and albumin level in stage IV NSCLC patients receiving first line (1L) IO.
Methods:
We identified 75 stage IV patients who received 1L IO therapy at USC Norris Comprehensive Cancer Center and Los Angeles General Medical Center from 2015 to 2022. The primary outcome was overall survival (OS) from time of IO with attention to pre-treatment BMI < 22, albumin < 3.5 g/dL, and PLR > 180.
Results:
Median age was 66.5 years with 49 (65.3%) males. 25 (33.3%) had BMI < 22. 45/75 (60%) had PLR > 180. Patients with BMI < 22 had inferior OS (13.1 months (m) vs. 37.4 m in BMI > 28, p-value = 0.042) along with patients with albumin<3.5 g/dL (OS: 2.8 m vs. 14.6 m, p-value = 0.0027), and patients with PLR>180 (OS: 8.7 m vs. 23.0 m, p = 0.028). Composite BMI < 22, PLR > 180 had the worst OS, p-value = 0.0331. Multivariate analysis controlling for age, smoking, gender, PD-L1 tumor proportion score (TPS), and histology (adenocarcinoma, squamous, adenosquamous, and large cell) showed that BMI (HR: 0.8726, 95% CI: 0.7892–0.954) and PLR > 180 (HR: 2.48, 95% CI: 1.076–6.055) were significant in OS mortality risk.
Conclusion:
Patients with a composite of BMI < 22, albumin < 3.5 g/dL, and PLR > 180 had significantly worse OS. This highlights the importance of screening for poor nutritional status and high PLR to better inform stage IV NSCLC patients receiving IO therapy of their prognosis and supportive care.
MicroAbstract:
We evaluated real-world prognostic utility of platelet lymphocyte ratio (PLR), body mass index (BMI), and albumin level in 75 Stage IV NSCLC patients receiving first line IO. Patients with a composite of BMI < 22, albumin < 3.5 g/dL, and PLR > 180 had significantly worse OS. This highlights the importance of screening for poor nutritional status and high PLR to better inform stage IV NSCLC patients of their prognosis and to emphasize supportive care needs.
Keywords: NSCLC, BMI, Platelet lymphocyte ratio, Cachexia, Real world
Introduction
Immune checkpoint inhibitors have revolutionized the care of patients with non-small cell lung cancer (NSCLC) and other malignancies, however it is important to consider which clinical and laboratory factors may predict a response to immunotherapy (IO) in order to better prognosticate these patients. Currently, the only approved biomarkers to predict response to IO in NSCLC in the clinical setting is the programmed cell death ligand-1(PD-L1)/programmed cell death protein-1 (PD-1) expression and tumor mutational burden (TMB) [1,2]. Several studies including meta-analyses have proposed an additional biomarker-platelet lymphocyte ratio (PLR) as a potential predictor of IO response in NSCLC patients. These studies reported that elevated pre-treatment PLR showed worse outcomes in patients who received IO [3–6]. PLR is simple to calculate from a patient’s complete blood count with differential (CBC), which is a relatively low-cost test done in all patients prior to initiating IO [7,8]. PLR can aid in prognosticating NSCLC cancer patients who are on immune checkpoint inhibitors [3,9,10]. The mechanism by which elevated PLR can decrease IO response is not fully understood, however, PLR is a marker of a pro-inflammatory state, which promotes tumorigenesis and can significantly alter the tumor microenvironment [11,12]. Platelet derived nucleotides can promote tumor-cell transendothelial migration and metastasis through the P2Y2 receptor [13]. Furthermore, tumor-induced platelet activation and platelet-induced growth and dissemination can trigger thrombosis and amplify a tumor [14]. In addition, platelets secrete platelet-derived growth factor (PDGF), platelet activating factor (PAF), and vascular endothelium growth factor (VEGF), all of which can support angiogenesis and tumor growth [15]. (PDGF has been shown to be involved in the acceleration of growth in metastatic breast tumors [16]. VEGF consists of three receptor kinases that are structured similar and all have a role in possible angiogenesis—VEGFR1 has been implicated in the involvement of in hematopoesis, migration of monocytes, and recruitment of bone marrow-derived progenitor cells, VEGFR2 functions include endothelial cell survival, migration, and vascular permeability while VEGFR3 functions in the remodeling of embryonic primary capillary plexus and is involved in angiogenesis and lymphangiogenesis [17]. Studies suggest that the blood platelets interact with lung cancer cells and PD-L1 protein can be transferred from NSCLC cells to platelets in a fibronectin 1, integrin α5β1, and GPIbα dependent manner, and platelets that express PD-L1 may inhibit the infiltration of CD4+ and CD8+ cells into the tumor microenvirnoment [18]. While there have been multiple studies looking at the prognostic utility of high PLR, there have not been any notable studies looking at its clinical application. Thus, this suggests that another clinical marker should be used in conjunction with high PLR to provide clinical guidance towards therapy or supportive care.
Another clinical factor that is associated with outcomes in NSCLC patients is nutritional status, which can be estimated with body mass index (BMI) and albumin levels. Unlike with other solid tumors, overweight or obese NSCLC patients have been found to have better outcomes though this has not been well understood [19–22]. One study specifically investigating NSCLC patients on IO showed that the median time to progression was significantly longer in overweight and obese patients [23]. A study by Liu and colleagues found that patients with BMI > 25 and albumin >3.7 g/L had a longer progression free survival (PFS) compared to those with BMI and albumin levels below those cutoffs [24]. In contrast, cachexic NSCLC patients have been found to have worse outcomes [25]. Cancer cachexia syndrome (CCS) is a pro-inflammatory state that involves poor nutritional status, significant weight loss through catabolism of adipose tissue and skeletal muscle, and has been associated with worse survival and response to therapy [26]. Cancer cachexia is defined as weight loss of greater than five percent over six months, or weight loss of greater than two percent in individuals already showing depletion based on a BMI < 20 kg/m2 or sarcopenia [27]. Specifically, CCS has been associated with poorer response to IO, and associated with worse PFS and overall survival (OS) [28]. BMI and albumin levels are both markers of nutritional status and can aid in identifying those with poor nutritional status or CCS [24]. Current recommendations for nutrition guidance in patients include engaging in regular physical activity if feasible and adopting a prudent diet along with checking for deficiencies of vitamins particularly vitamin D which has been suggested to be needed in optimizing the effectiveness of protein supplements [29,30].
Assessing the real-world application of nutritional status and laboratory factors including BMI, albumin, and PLR in metastatic NSCLC is important in order to estimate survival in patients receiving IO in the clinical environment and guide supportive care, particularly in cachexic patients prior to IO initiation. While previous studies have investigated PLR and nutritional status in various malignancies, to our knowledge this is the first retrospective study that specifically investigates real-world, 1st line treatment data in stage IV NSCLC patients who lack actionable mutations. Furthermore, the use of these measurements which are readily available in the clinical setting can help guide clinicians with management of supportive care and help quantify prognosis with survival data. With the significant costs of cancer, it is imperative to optimize use of standard labs and assessments in the clinic and thus, this study provides valuable information that clinicians can share with their patients based on standard labs and BMI assessment in the clinic. This retrospective, dual-center study in the U.S.A. investigates the utility of PLR, BMI, and albumin level in predicting the OS and PFS in a population of metastatic NSCLC patients who were treated with IO therapy.
Patients and methods
Patient population
This is a retrospective study of all adult patients diagnosed with NSCLC who were treated at Los Angeles General Medical Center (LA General) and University of Southern California Norris Comprehensive Cancer Center (Norris) from 01/01/2015 to 12/31/2022. The study was approved by the University of Southern California Institutional Review Board (IRB). Given the retrospective nature of the study, informed consent was waived.
Data was manually abstracted from patient electronic medical records and entered into a password protected data file. Data was captured and reviewed in a secure institutional file and only IRB approved study personnel had access to the data.
Primary outcomes included PFS and OS from time of IO. We compared patients with pre-treatment IO BMI < 22, BMI 22–28 vs. BMI > 28, albumin < 3.5 g/dL vs. >3.5 g/dL, and PLR > 180 vs. PLR < 180.
Clinical characteristics
The patients included in the study were patients with a diagnosis of NSCLC treated either at LA General or Norris. We selected patients with stage IV NSCLC who received 1L IO therapy. Patients who had epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), or proto-oncogene tyrosine kinase-1 (ROS1) mutations were excluded along with those who had previously received IO therapy. Smoking history was obtained from chart review. Date of initial diagnosis was recorded as the first date of confirmatory pathology whether from curative surgery or biopsy. BMI was obtained from chart review at 2 months before diagnosis, at diagnosis, and at time of initiation of IO. PLR and albumin were obtained at time of diagnosis and at time of initiation of IO. Time from initiation of IO therapy to last contact was defined as date of first dose of IO therapy to date of last clinical encounter (data cutoff date of December 31, 2022). Time from initial IO therapy to death was defined as documented date of death on the chart.
Statistical analysis
Overall survival (OS) was the period from initiation of IO therapy to death due to any cause. OS was censored at the date of last contact if the patient was still alive. Progression free survival (PFS) was defined as the period from initiation of IO therapy to documented date of progression. OS and PFS were estimated with the Kaplan-Meier method with log-rank test [31]. Multivariate Cox hazards regression was used to determine the risk of BMI (continuous variable) and PLR > 180 (yes, no) in OS adjusting for age at diagnosis (continuous variable), gender (female, male), smoking history (yes if current/former, no if never smoker), PD-L1 tumor proportion score ≥1% (yes, no), albumin < 3.5 g/dL (yes, no), and nonsquamous histology (yes if adenocarcinoma, large cell, no if squamous, adenosqaumous) [32]. Statistical analysis was conducted with Graphpad Prism 9 software [31,32].
Results
Patient characteristics
A total of 515 NSCLC patients treated at LA General Medical Center and University of Southern California Norris Comprehensive Cancer Center were reviewed. 75 patients with stage IV NSCLC without EGFR, ALK, and ROS1 mutations who received IO therapy were determined to be eligible for this retrospective study (Table 1). The median age at diagnosis was 66.3 years old and the median age at initiation of immunotherapy was 66.5 years old. The majority of patients were males (65.3%). The majority (66.7%) had lung adenocarcinoma, while squamous cell carcinoma (29.3%) was the second most represented histologic subtype. When examining mutation status, 17.3% had a KRAS mutation. When examining PD-L1 status, 25.3% of patients had a tumor proportion score (TPS) of 1–49 percent, while an additional 29.3% of patients had TPS of 50 percent or greater. Most patients were former smokers (64.0%), whereas 9.3% were current smokers.
Table 1.
Patient characteristics.
| BMI at start of immunotherapy | Platelet/Lymphocyte ratio at start of immunotherapy | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | BMI < 22 | BMI 22–28 | BMI > 28 | PLR < 180 | PLR > 180 | |
| Median Age at Diagnosis | 66.3 | 68.2 | 67.2 | 67.4 | 67.9 | |
| Median Age at Start of Immunotherapy | 66.5 | 69.4 | 68.6 | 66.2 | 66.5 | |
| Gender (%) | Male | 14 (18.7) | 24 (32) | 11 (14.7) | 23 (30.7) | 26 (34.7) |
| Female | 11 (14.7) | 8 (10.7) | 7 (9.3) | 7 (9.3) | 19 (25.3) | |
| Smoking Status (%) | Current | 1 (1.3) | 4 (5.3) | 2 (2.7) | 3 (4) | 4 (5.3) |
| Former | 20 (26.7) | 18 (24) | 10 (13.3) | 23 (30.7) | 25 (33.3) | |
| Never | 4 (5.3) | 10 (13.3) | 6 (8) | 4 (5.3) | 16 (21.3) | |
| Histology (%) | Adenocarcinoma | 17 (22.7) | 21 (28) | 12 (16) | 21 (28) | 29 (38.7) |
| Squamous | 6 (8) | 10 (13.3) | 6 (8) | 9 (12) | 13 (17.3) | |
| Adenosquamous | 1 (1.3) | 1 (1.3) | 0 | 0 | 2 (2.7) | |
| Large Cell | 1 (1.3) | 0 | 0 | 0 | 1 (1.3) | |
| PD-L1 Tumor Proportion Score (%) | <1% | 8 (10.7) | 14 (18.7) | 7 (9.3) | 10 (13.3) | 19 (25.3) |
| 1–49% | 8 (10.7) | 5 (6.7) | 6 (8) | 8 (10.7) | 11 (14.7) | |
| 50+% | 9 (12) | 9 (12) | 4 (5.3) | 10 (13.3) | 12 (16) | |
| Missing | 5 (6.7) | 5 (6.7) | ||||
| KRAS Mutation (%) | Yes | 5 (29.4) | 5 (29.4) | 3 (17.6) | 6 (35.3) | 7 (41.2) |
| KRAS G12C | 2 (11.8) | 1 (5.9) | 1 (5.9) | 2 (11.8) | 2 (11.8) | |
Of 70 records available with PLR information, 30 had PLR < 180 (42.9%) and 40 had PLR > 180 (57.1%) at initiation of IO. Of 75 patients, 42 had progressive disease while on IO (56%), 30 patients did not have evidence of progression of disease (40%), while the remaining 4% was unknown. The most common initial IO used was pembrolizumab (80%) and 43/75 (57.3%) used chemotherapy in addition to IO at therapy initiation.
BMI, PLR in OS and PFS
Patients with a BMI < 22 at the start of IO had an inferior median OS (BMI < 22: 13.1 m vs. BMI > 28: 37.4 m, p-value = 0.0420 with a trend towards worse median PFS BMI < 22: 6.2 m vs. BMI > 28: 16 m, p-value = 0.4781). Patients with PLR > 180 had an inferior median PFS 4.4 m vs. 11.4 m, p-value = 0.025 and median OS 8.7 m vs. 23.0 m, p-value = 0.0277).
A composite of BMI and PLR showed that in PFS analysis, BMI < 22 and PLR > 180 had the worst PFS of 3.1 m while BMI > 28 and PLR < 180 had the best PFS of 16.0 m (p-value = 0.1824) (Fig. 1A, Table 2).
Fig. 1A.
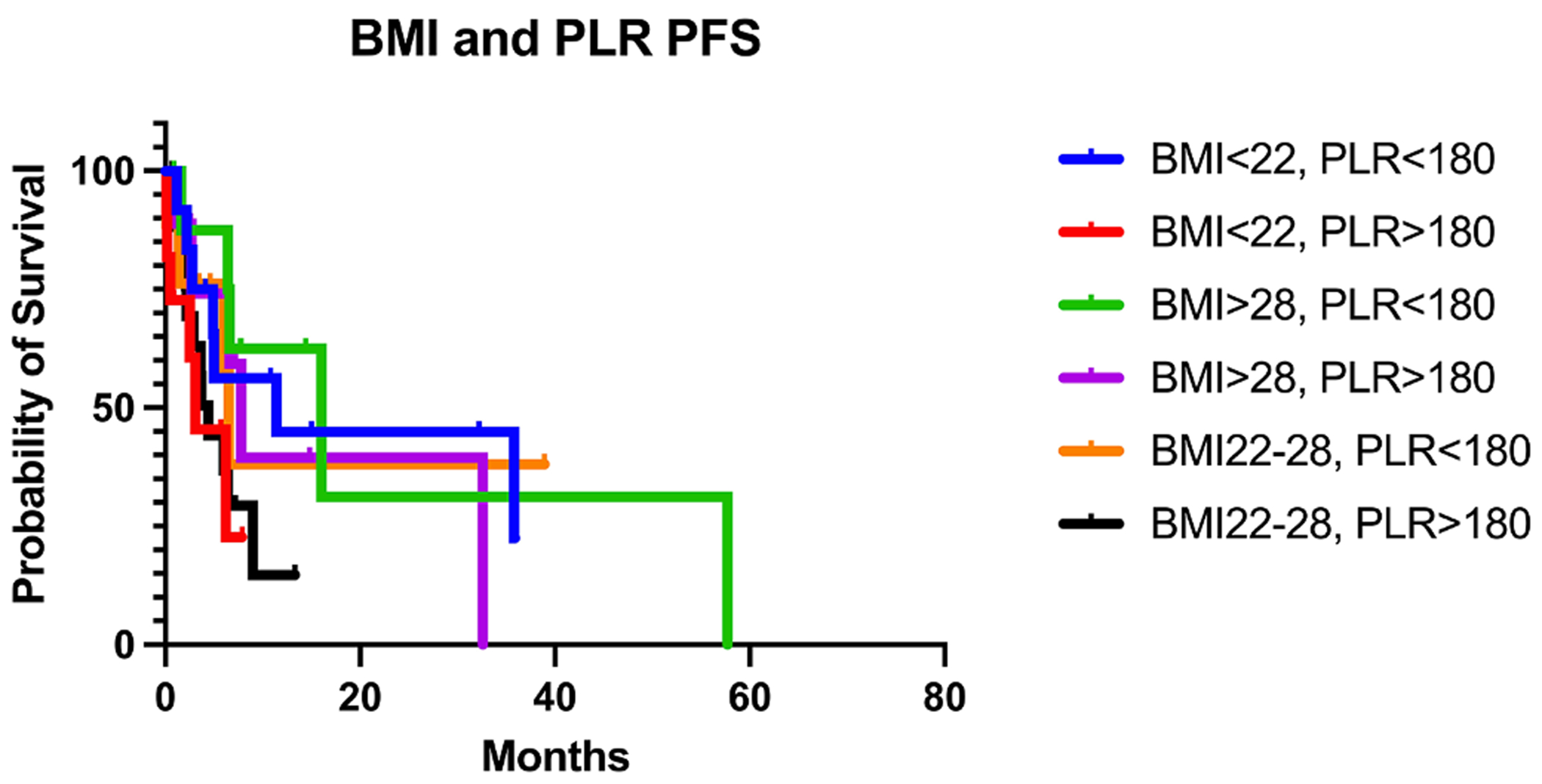
PFS of 1L IO stratified by composite of BMI and PLR at initiation of IO.
Table 2.
Composite of BMI and PLR at initiation of IO- PFS and OS.
| BMI < 22, PLR > 180 | BMI < 22, PLR < 180 | BMI 22–28 PLR > 180 | BMI 22–28 PLR < 180 | BMI > 28, PLR > 180 | BMI > 28, PLR < 180 | |
|---|---|---|---|---|---|---|
| N | 11 | 12 | 19 | 9 | 9 | 9 |
| Median PFS (m) | 3.1 | 11.4 | 4.4 | 6.5 | 7.8 | 16.0 |
| p-value (log-rank) | 0.1824 | |||||
| Median OS (m) | 8.7 | 22.2 | 8.7 | 9.6 | 37.4 | 57.7 |
| p-value (log-rank) | 0.0331 |
A composite of BMI < 22 and BMI 22–28 and PLR > 180 had the worst median OS of 8.7 m while, patients with BMI > 28 and PLR < 180 had a median OS 57.7 m (p-value = 0.0331) (Fig. 1B, Table 2).
Fig. 1B.
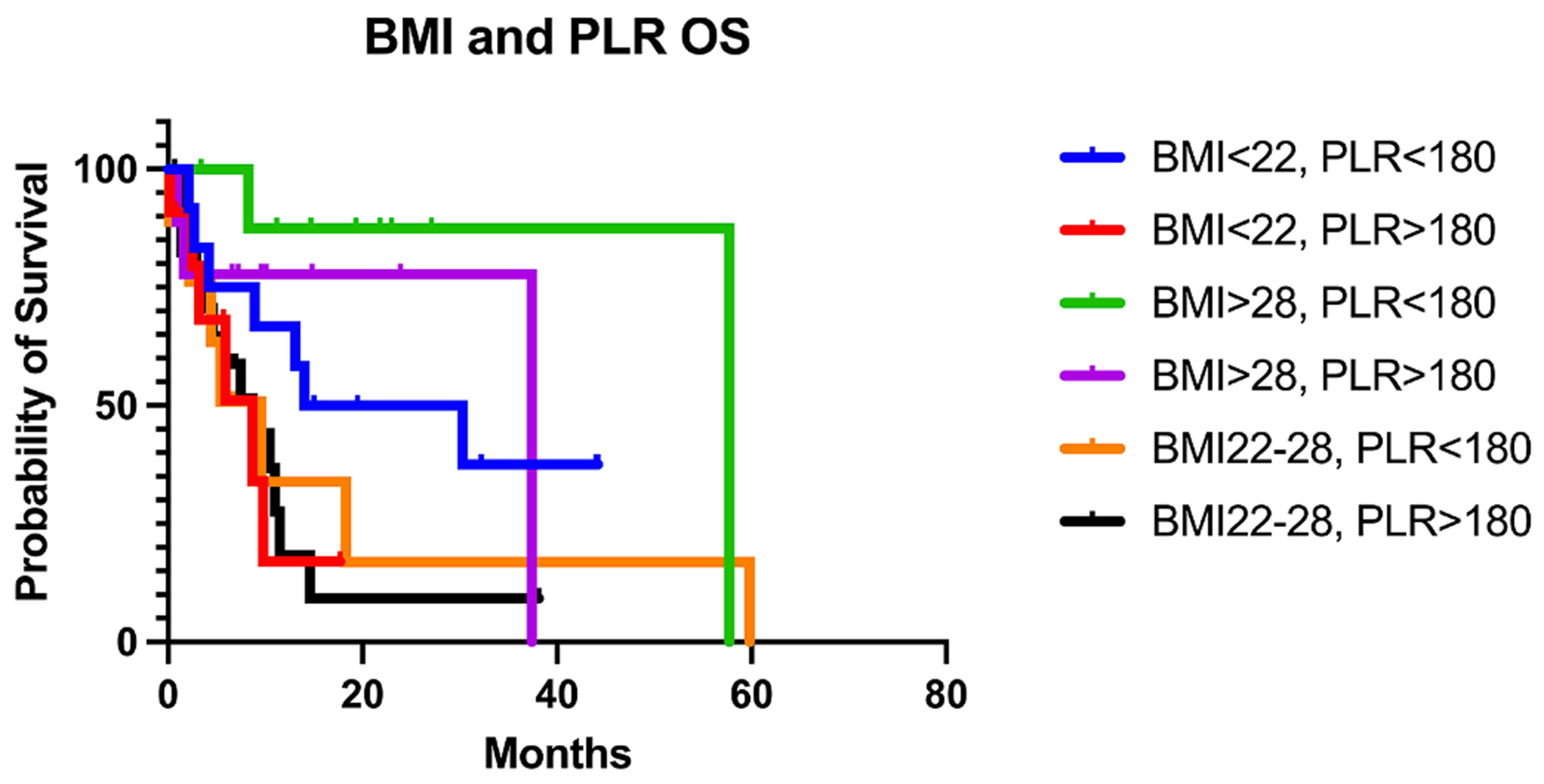
OS of 1L IO stratified by composite of BMI and PLR at initiation of IO.
Change in BMI and albumin and OS
Patients who lost one or more BMI point during two months prior to IO initiation had a worse OS (5.9 m) than patients who lost less than one BMI point (14 m), p-value = 0.0481 (Fig. 2A, Table 3). There were 64 patients with documented albumin levels at the start of IO. 15 patients had albumin <3.5 g/dL (23.4%), while 49 had albumin >3.5 g/dL (76.6%). Patients with albumin level <3.5 g/dL at time of IO initiation showed inferior OS to patients with albumin >3.5 g/dL at time of IO initiation albumin<3.5 g/dL: 2.8 m vs albumin >3.5 g/dL: 14.6 m, p-value = 0.0027 (Fig. 2B, Table 4). Patients with an albumin level <3.5 g/dL showed a trend of inferior PFS compared to patients with albumin >3.5 g/dL (6.4 m vs 9 m, p value = 0.1288).
Fig. 2A.
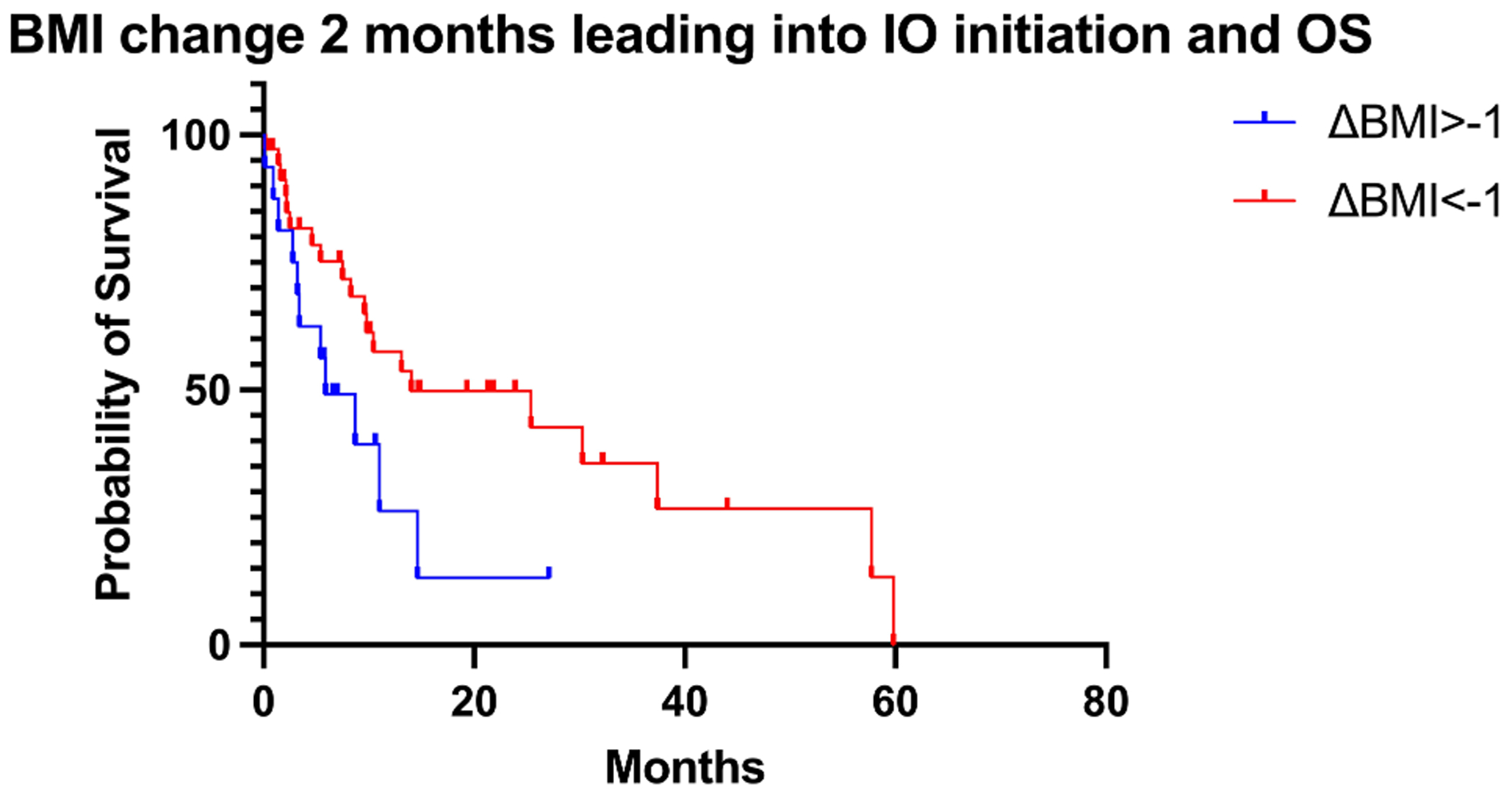
BMI change 2 months leading into IO initation and OS.
Table 3.
Change in BMI at two months prior to initiation of IO and OS.
| ΔBMI > −1 | ΔBMI < −1 | |
|---|---|---|
| N | 16 | 36 |
| Median OS (m) | 5.9 | 14 |
| P-value (log-rank) | 0.0481 |
Fig. 2B.
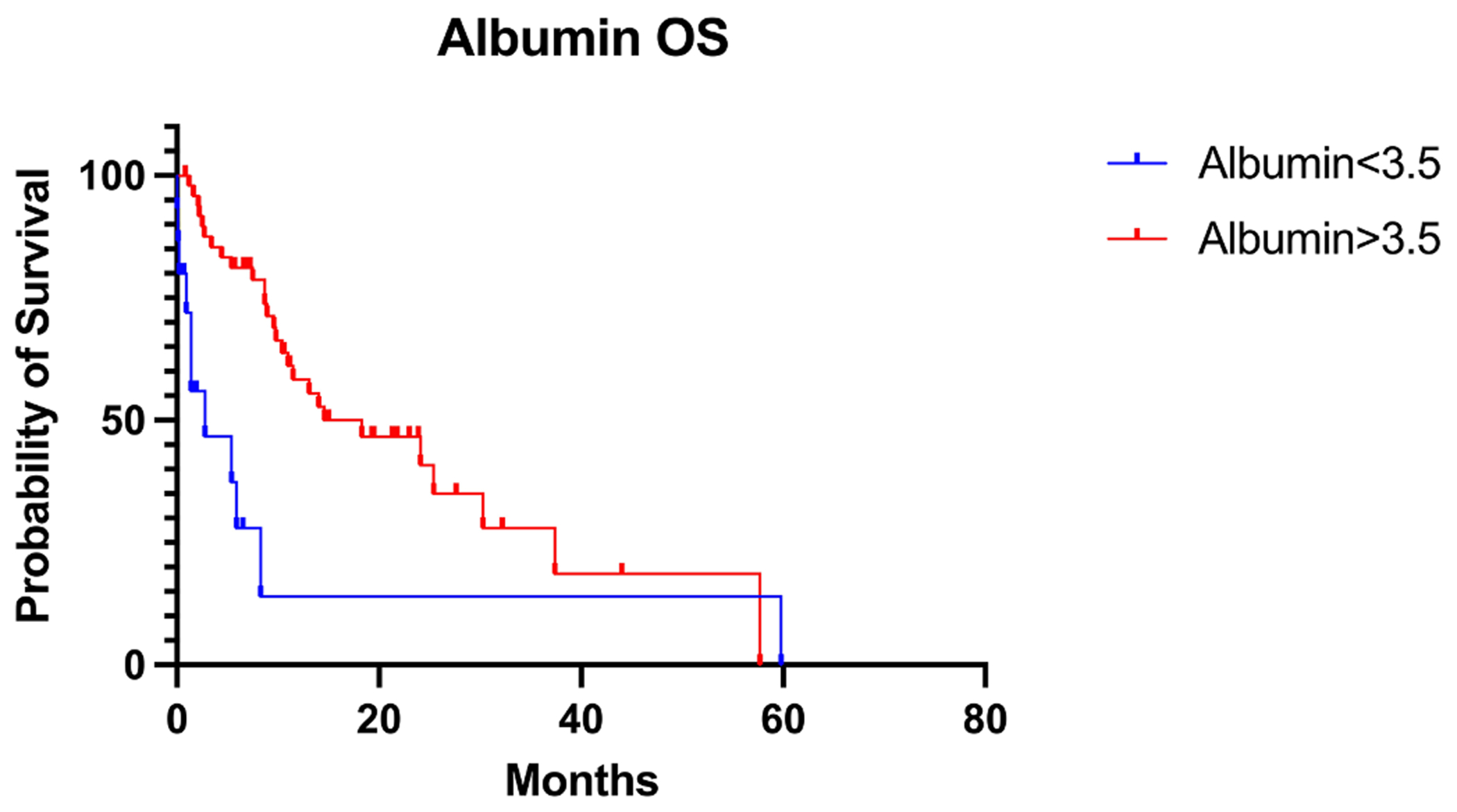
Albumin level at IO initiation and OS.
Table 4.
Albumin level at IO Initiation and OS and PFS.
| Albumin < 3.5 d/L | Albumin > 3.5 g/dL | |
|---|---|---|
| N | 15 | 49 |
| Median OS (m) | 2.8 | 14.6 |
| P-value (log-rank) | 0.0027 | |
| N | 15 | 49 |
| Median PFS (m) | 6.4 | 9 |
| P-value (log-rank) | 0.1288 |
Multivariate analysis
Multivariate analysis for OS controlling for age at diagnosis, gender, smoking history, PD-L1 tumor proportion score ≥1%, albumin <3.5 g/dL, and nonsquamous histology showed that BMI (HR: 0.8726, 95% CI: 0.7892–0.954) and PLR>180 (HR: 2.48, 95% CI: 1.076–6.055) were significant variables in OS mortality risk with albumin<3.5 g/dL also trending as a risk factor (HR: 2.50, 95% CI 0.9810–6.242), (Table 5).
Table 5.
Multivariate analysis of OS.
| Variable (n = 54) | Hazard ratio | 95% CI |
|---|---|---|
| Body Mass Index (BMI) (continuous variable) | 0.8726 | 0.7892 – 0.9540 |
| Platelet Lymphocyte Ratio (PLR)≥ 180 | ||
| Yes | 2.480 | 1.076 – 6.055 |
| No | REFERENCE | |
| Age at diagnosis (continuous variable) | 1.012 | 0.9800 – 1.049 |
| Sex | ||
| Female | 0.6391 | 0.1967 – 1.858 |
| Male | REFERENCE | |
| Smoking History (current or former) | ||
| Yes | 0.8759 | 0.2931 – 2.623 |
| No | REFERENCE | |
| Non-squamous Histology | ||
| No | 1.038 | 0.4234 – 2.437 |
| Yes | REFERENCE | |
| PD-L1 Tumor Proportion Score (TPS)≥ 1% | ||
| Yes | 0.9384 | 0.4224 – 2.151 |
| No | REFERENCE | |
| Albumin<3.5 g/dL | ||
| Yes | 2.495 | 0.9810 – 6.242 |
| No | REFERENCE |
Discussion
Our real-world study highlights easy to obtain clinical metrics that may be helpful in prognosticating patients in the clinical setting who are being initiated on IO for stage IV NSCLC, and brings further awareness to which patients may need additional supportive care. Our analysis showed inferior outcomes in patients particularly with the combination of PLR > 180 and BMI < 22.
High PLR is indicative of an inflammatory state, and several studies have noted worse outcomes in cancer patients with elevated PLR who are on IO [3,6,9,10,33]. A study by Diem and colleagues found that elevated PLR prior to treatment with nivolumab was associated with worse PFS and OS in patients with metastatic NSCLC [9]. Another study similarly found that high PLR was associated with worse OS and PFS, and additionally found that high PLR is associated with worse IO efficacy in metastatic NSCLC patients [4]. Our study similarly found that stage IV NSCLC patients with an elevated pre-treatment PLR (>180) had significantly inferior PFS and OS compared to those with lower pre-treatment PLR.
In addition to investigating PLR, we evaluated nutritional status on outcomes in our population by measuring pre-treatment albumin level, pre-treatment BMI, and change in BMI prior to IO initiation. In addition to finding that low pre-treatment BMI is associated with worse outcomes, our study also found that patients who dropped more than one BMI point two months prior to initiation of IO had worse OS compared to patients whose BMI dropped less than one point over the same time period. Our study also found that albumin level less than 3.5 g/dL is associated with significantly worse OS, indicating that poor pretreatment nutritional status negatively impacts OS. These conclusions are in line with the results of several studies which report that underweight or cachexic patients have worse outcomes on IO compared to the overweight population [25,28,34,35]. A study by Johannet and colleagues found that declining BMI prior to starting IO was associated with worse outcomes [36]. Similarly, a study by Magri and colleagues found that declining BMI between diagnosis and IO initiation, as well as low albumin level were associated with worse OS [37].
There are several proposed mechanisms by which cachexia contributes to decreased response to IO in NSCLC patients [34]. Inflammatory markers including interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor (TNF)-α are upregulated in cachexia and suppress tumor-infiltrating lymphocytes (TILs) which regulate the PDL-1 inhibitor anti-tumor response [34,38,39]. Interestingly, overweight and obese patients on IO have the best outcomes compared to patients in other weight groups [40,41]. The mechanism in which obesity improves survival in those on IO is poorly understood but it is thought that changes in adipose tissue of obese individuals lead to a reduction of regulatory T cells, increased fatty acid reflux, vascularization, hypoxia, and increased leptin production. This in turn leads to increases of various immune infiltrates in the tumor microenvironment including CD4+ and CD8+ T cells in the adipose tissue. One theory that has been suggested is an increase of Major Histocompatability Complex-II by adipocytes via a leptin-dependent mechanism [42,43]. Leptin has been associated with elevated programmed cell death protein-1 (PD1) levels and may help explain why obesity may increase PD1/PD-L1 blockade leading towards better outcomes with IO in obese individuals [41,44]. A study by Collet and colleagues specifically investigated patients on IO for metastatic cancer and found that patients who were overweight with BMI>25 had a higher OS [40]. Our study results are consistent with these findings in that obese individuals seemed to fare better with first line IO than patients with low BMIs.
Based on the results of our study, we conclude that these parameters may be useful criteria for screening patient populations for interventions targeting cachexia. Current strategies to manage cachexia in advanced cancer include progesterone analogs such as megestrol acetate and glucocorticoids when utilized for a limited duration [45]. Anamorelin, a ghrelin agonist, has been studied in clinical trials but was not licensed in the U.S.A. due to lack of adequate data on patient benefits and safety [46]. Other novel targets include growth differentiation factor-15 (GDF-15) and fibroblast growth factor inducible-14 (Fn14) [47,48]. Dietary counseling with or without the intervention of oral nutritional supplements have been shown to increase body weight [49]. Enteral or parenteral feeding are not routinely recommended. [50] Around the world, there are ongoing clinical trials evaluating efficacy of physical activities and nutritional support. The Nutritional and Exercise Treatment for Advanced Center (NEXTAC) study is ongoing in Japan and is looking at efficacy of early nutritional and exercise interventions in older patients with advanced NSCLC and pancreatic cancer. The phase 1 results showed significant differences in physical activity, quality of life, and mental activity in those who were compliant with the planned program [51]. The Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy (MENAC) study is another trial that recently completed with results pending [52].
A strength of our study is that the data originates from real-world patient data, as opposed to clinical trial data, in advanced NSCLC. This is important as our study focuses on patients with poor nutritional status, an understudied population, as these patients may not have the requisite performance status for a clinical trial. Another strength of our study is our composite data showing that lower BMI and PLR > 180 had the worst OS of all patients in the studied population. Several studies independently report these factors and their association with survival, however there is limited research on these clinical factors being analyzed concomitantly and in the real-world setting. Patients with both low BMI and high PLR are at risk for worse outcomes and may need closer monitoring during treatment. Future, prospective clinical studies that evaluate stage IV NSCLC IO patients with low BMI, low albumin, and high PLR and focus on the effects of nutritional and physical activity intervention are warranted. Finally, there are many factors studied in NSCLC such as pathology, mutations and corresponding comutations, and possible molecular patterns of resistance yet these biomarkers ignore the whole person and the role of the patient as the substrate on which the disease acts. Our results demonstrate the greater impact of how important a patient’s general health is when considering whether a treatment will work.
Our study has limitations, with the most notable being the retrospective nature of the study, which highlights the need for more prospective, supportive care studies with interventions for this patient population. Another limitation to our study includes the small sample size. Given the inclusion criteria of the study, the 515 reviewed charts yielded a study population of 75 eligible patients. It is possible that a higher sample size may have yielded a significant result in our PFS data and also allowed us to do Kaplan-Meier analysis on NSCLC patients that had low BMI, low albumin, and high PLR. Finally, we were limited by the scope of the clinical data available. Many stage IV NSCLC patients with cancer cachexia present with months of weight loss and decreased nutrition support prior to IO initiation, thus it would have been beneficial to have data on more patients several months prior to IO initiation. However, our results show a significant difference in survival in patients with low BMI, low albumin, and high PLR, and given the easy accessibility of obtaining these values, there is potential for universal clinical application.
Conclusion
Our study concludes that patients being initiated on IO for stage IV NSCLC had worse OS when pre-treatment BMI < 22, albumin < 3.5 g/dL, or PLR > 180. Patients with weight loss of more than one BMI point, within two months of initiating immunotherapy had worse OS than those without this significant of weight loss. This real-world study provides objective survival data highlighting the importance of pretreatment screening for high PLR and poor nutritional status in patients who are being initiated on IO for stage IV NSCLC to better inform patients of their prognosis and to emphasize the need of better supportive care in patients with low BMI, high PLR, and low albumin. We hope these conclusions prompt future studies which may target interventions in patients with poor nutritional status or cachexia, with the goal of improving outcomes in those with advanced NSCLC patients who are being initiated on IO.
Clinical practice points.
We recommend pre-treatment screening of PLR, as PLR>180 is associated with worse overall survival in this patient population.
We recommend pre-treatment screening for poor nutritional status with BMI and albumin level prior to starting immunotherapy, as pretreatment BMI < 22 and albumin level <3.5 g/dL were associated with worse overall survival in patients being treated with immunotherapy for NSCLC.
Pre-treatment screening may help with early interventions to improve worse outcomes, though further prospective studies on supportive care interventions are needed.
Acknowledgments
We thank the patients and their families involved in the care of the patients who were part of this retrospective study.
Funding
This work was supported in part by the USC Norris Comprehensive Cancer Center (Core) Grant, P30 CA014089.
Abbreviations:
- BMI
body mass index
- OS
overall survival
- PFS
progression free survival
- PLR
platelet lymphocyte ratio
- 1L
first line
- IO
immunotherapy
- NSCLC
non-small cell lung cancer
- m
month
- TPS
tumor proportion score
- CBC
complete blood count
- CCS
cancer cachexia syndrome
- LA General
Los Angeles General Medical Center
- IRB
Institutional Review Board
- EGFR
epidermal growth factor receptor
- ALK
anaplastic lymphoma kinase
- ROS1
proto-oncogene tyrosine kinase-1
- IL-6
interleukin-6
- IL-1B
interleukin-1 beta
- TNF-α
tumor necrosis factor alpha
- TILs
tumor-infiltrating lymphocytes
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death ligand-1
- TMB
tumor mutational burden
- PDGF
platelet-derived growth factor (PDGF)
- PAF
platelet activating factor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- GDF-15
growth differentiation factor-15
- Fn14
fibroblast growth factor inducible-14
- NEXTAC
The Nutritional and Exercise Treatment for Advanced Center Study
- MENAC
Multimodal Intervention for Cachexia in Advanced Cancer Patients Undergoing Chemotherapy Study
Footnotes
Informed consent
Informed consent was waived by the University of Southern California IRB due to the retrospective nature of the study. All patient data was de-identified prior to use.
Declaration of generative AI in scientific writing
N/A
CRediT authorship contribution statement
Madeline MacDonald: Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. Darin Poei: Data curation, Formal analysis, Investigation, Writing – review & editing. Alexis Leyba: Data curation, Formal analysis, Investigation, Writing – review & editing. Raymond Diep: Data curation, Formal analysis, Methodology, Software, Investigation, Writing – review & editing. Krithika Chennapan: Data curation, Formal analysis, Writing – review & editing. Christopher Leon: Data curation, Writing – review & editing. Bing Xia: Writing – review & editing. Jorge J. Nieva: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Robert Hsu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
Dr. Xia is an employee of Janssen Research and Development. Dr. Nieva receives personal fees from ANP Technologies, Aadi Biosciences, G1 Therapeutics, Astra Zeneca, Fujirebio and Naveris. He receives research support from Merck and Genentech, and has ownership in Cansera, Epic Sciences, Quantgene, and Indee. Dr. Hsu is a consultant for Targeted Oncology and has received honoraria from DAVA Oncology and The Dedham Group.
All other authors do not have any declarations of interest.
References
- [1].Meng X, Huang Z, Teng F, Xing L, Yu J, Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy, Cancer Treat. Rev 41 (10) (2015) 868–876. [DOI] [PubMed] [Google Scholar]
- [2].Marabelle A, Fakih M, Lopez J, et al. , Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study, Lancet Oncol. 21 (10) (2020) 1353–1365. [DOI] [PubMed] [Google Scholar]
- [3].Zhang N, Jiang J, Tang S, Sun G, Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis, Int. Immunopharmacol 85 (2020), 106677. [DOI] [PubMed] [Google Scholar]
- [4].Kartolo A, Holstead R, Khalid S, et al. , Serum neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in prognosticating immunotherapy efficacy, Immunotherapy 12 (11) (2020) 785–798. [DOI] [PubMed] [Google Scholar]
- [5].Zhou K, Cao J, Lin H, et al. , Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: a systematic review and meta-analysis, Front. Oncol 12 (2022), 962173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang H, Li C, Yang R, Jin J, Liu D, Li W, Prognostic value of the platelet-to-lymphocyte ratio in lung cancer patients receiving immunotherapy: a systematic review and meta-analysis, PLoS ONE 17 (5) (2022), e0268288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ettinger DS, Wood DE, Aisner DL, et al. , Non-small cell lung cancer, version 3.2022, NCCN Clinical practice guidelines in oncology, J. Natl. Compr. Canc. Netw 20 (5) (2022) 497–530. [DOI] [PubMed] [Google Scholar]
- [8].Chabot-Richards DS, George TI, White blood cell counts: reference methodology, Clin. Lab. Med 35 (1) (2015) 11–24. [DOI] [PubMed] [Google Scholar]
- [9].Diem S, Schmid S, Krapf M, et al. , Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab, Lung Cancer 111 (2017) 176–181. [DOI] [PubMed] [Google Scholar]
- [10].Platini H, Ferdinand E, Kohar K, et al. , Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic markers for advanced non-small-cell lung cancer treated with immunotherapy: a systematic review and meta-analysis, Medicina (Kaunas) 58 (8) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu J, Li S, Zhang S, et al. , Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab, J. Clin. Lab. Anal 33 (8) (2019) e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Greten FR, Grivennikov SI, Inflammation and cancer: triggers, mechanisms, and consequences, Immunity 51 (1) (2019) 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S, Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor, Cancer Cell 24 (1) (2013) 130–137. [DOI] [PubMed] [Google Scholar]
- [14].Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M, The platelet-cancer loop, Eur. J. Intern. Med 24 (5) (2013) 393–400. [DOI] [PubMed] [Google Scholar]
- [15].Zhu Y, Si W, Sun Q, Qin B, Zhao W, Yang J, Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer, Oncotarget 8 (1) (2017) 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ariad S, Seymour L, Bezwoda WR, Platelet-derived growth factor (PDGF) in plasma of breast cancer patients: correlation with stage and rate of progression, Breast Cancer Res. Treat 20 (1) (1991) 11–17. [DOI] [PubMed] [Google Scholar]
- [17].Sullivan LA, Brekken RA, The VEGF family in cancer and antibody-based strategies for their inhibition, MAbs 2 (2) (2010) 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hinterleitner C, Strahle J, Malenke E, et al. , Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer, Nat. Commun 12 (1) (2021) 7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xie HJ, Zhang X, Wei ZQ, Long H, Rong TH, Su XD, Effect of body mass index on survival of patients with stage I non-small cell lung cancer, Chin. J. Cancer 36 (1) (2017) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lam VK, Bentzen SM, Mohindra P, et al. , Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC), Lung Cancer 104 (2017) 52–57. [DOI] [PubMed] [Google Scholar]
- [21].Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL, Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials, Cancer Epidemiol., Biomark. Prevent 26 (1) (2017) 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pilikidou M, Palyvou F, Papadopoulou SK, et al. , Lung cancer, treatment and nutritional status, Mol. Clin. Oncol 15 (6) (2021) 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gelibter A, Occhipinti M, Pisegna S, Cortellini A, Cortesi E, Marchetti P, Status of correlation between BMI and response to immunocheck-point inhibitor in advanced non-small-cell lung cancer, Lung Cancer Manag 9 (2) (2020) LMT26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu Z, Diao Y, Li X, Body mass index and serum markers associated with progression-free survival in lung cancer patients treated with immune checkpoint inhibitors, BMC Cancer 22 (1) (2022) 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Turcott JG, Martinez-Samano JE, Cardona AF, et al. , The role of a cachexia grading system in patients with non-small cell lung cancer treated with immunotherapy: implications for survival, Nutr. Cancer 73 (5) (2021) 794–801. [DOI] [PubMed] [Google Scholar]
- [26].Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH, Cancer-associated cachexia, Nat. Rev. Dis. Primers 4 (2018) 17105. [DOI] [PubMed] [Google Scholar]
- [27].Fearon K, Strasser F, Anker SD, et al. , Definition and classification of cancer cachexia: an international consensus, Lancet Oncol 12 (5) (2011) 489–495. [DOI] [PubMed] [Google Scholar]
- [28].Rounis K, Makrakis D, Tsigkas AP, et al. , Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study, Transl. Lung Cancer Res 10 (8) (2021) 3538–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ravasco P, Nutrition in cancer patients, J. Clin. Med 8 (8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arends J, Bachmann P, Baracos V, et al. , ESPEN guidelines on nutrition in cancer patients, Clin. Nutr 36 (1) (2017) 11–48. [DOI] [PubMed] [Google Scholar]
- [31].Motulsky HJ GraphPad prism 9 statistics guide - Kaplan-Meier (nonparametric) survival analysis. https://www.graphpad.com/guides/prism/latest/statistics/stat_kaplan-meier_survival_analysis.htm. Published 2023. Accessed Accessed May 22, 2023.
- [32].Motulsky HJ GraphPad prism 9 statistics guide - cox regression (Cox proportional hazards model). https://www.graphpad.com/guides/prism/latest/statistics/stat_cox_regression_model.htm. Accessed Accessed May 22, 2023.
- [33].Mandaliya H, Jones M, Oldmeadow C, I.I. Nordman, Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI), Transl. Lung Cancer Res 8 (6) (2019) 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miyawaki T, Naito T, Kodama A, et al. , Desensitizing effect of cancer cachexia on immune checkpoint inhibitors in patients with advanced NSCLC, JTO Clin. Res. Rep 1 (2) (2020), 100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jo H, Yoshida T, Horinouchi H, et al. , Prognostic significance of cachexia in advanced non-small cell lung cancer patients treated with pembrolizumab, Cancer Immunol. Immunother 71 (2) (2022) 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Johannet P, Sawyers A, Qian Y, et al. , Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer, J. Immunother. Cancer 8 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Magri V, Gottfried T, Di Segni M, et al. , Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients, Cancer Manag. Res 11 (2019) 8201–8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bertrand F, Rochotte J, Colacios C, et al. , Blocking tumor necrosis factor alpha enhances CD8 T-cell-dependent immunity in experimental melanoma, Cancer Res 75 (13) (2015) 2619–2628. [DOI] [PubMed] [Google Scholar]
- [39].Flint TR, Janowitz T, Connell CM, et al. , Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity, Cell Metab 24 (5) (2016) 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Collet L, Delrieu L, Bouhamama A, et al. , Association between body mass index and survival outcome in metastatic cancer patients treated by immunotherapy: analysis of a French Retrospective Cohort, Cancers (Basel) 13 (9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang Z, Aguilar EG, Luna JI, et al. , Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade, Nat. Med 25 (1) (2019)141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL, The effects of obesity on anti-cancer immunity and cancer immunotherapy, Cancers (Basel) 12 (5) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferrante AW Jr., The immune cells in adipose tissue, Diabetes Obes. Metab 15 Suppl 3 (03) (2013) 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dai X, Bu X, Gao Y, et al. , Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade, Mol. Cell 81 (11) (2021) 2317–2331. e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morita-Tanaka S, Yamada T, Takayama K, The landscape of cancer cachexia in advanced non-small cell lung cancer: a narrative review, Transl. Lung Cancer Res 12 (1) (2023) 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Temel JS, Abernethy AP, Currow DC, et al. , Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials, Lancet Oncol 17 (4) (2016) 519–531. [DOI] [PubMed] [Google Scholar]
- [47].Tsai VW, Brown DA, Breit SN, Targeting the divergent TGFbeta superfamily cytokine MIC-1/GDF15 for therapy of anorexia/cachexia syndromes, Curr. Opin. Support Palliat. Care 12 (4) (2018) 404–409. [DOI] [PubMed] [Google Scholar]
- [48].Cao Z, Burvenich IJ, Zhao K, et al. , Identification of potential biomarkers for cancer cachexia and anti-Fn14 therapy, Cancers (Basel) 14 (22) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Naito T, Mitsunaga S, Miura S, et al. , Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer, J. Cachexia Sarcopenia Muscle 10 (1) (2019) 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Roeland EJ, Bohlke K, Baracos VE, et al. , Management of cancer cachexia: ASCO guideline, J. Clin. Oncol 38 (21) (2020) 2438–2453. [DOI] [PubMed] [Google Scholar]
- [51].Miura S, Naito T, Mitsunaga S, et al. , A randomized phase II study of nutritional and exercise treatment for elderly patients with advanced non-small cell lung or pancreatic cancer: the NEXTAC-TWO study protocol, BMC Cancer 19 (1) (2019) 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Solheim TS, Laird BJA, Balstad TR, et al. , Cancer cachexia: rationale for the MENAC (Multimodal-exercise, nutrition and anti-inflammatory medication for cachexia) trial, BMJ Support. Palliat. Care 8 (3) (2018) 258–265. [DOI] [PubMed] [Google Scholar]


