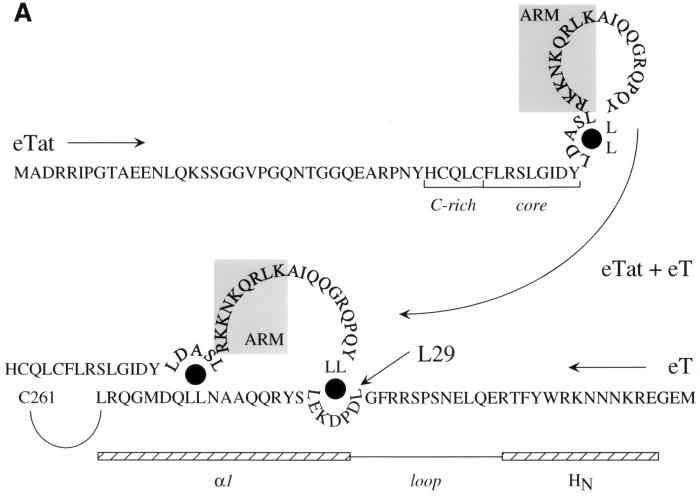
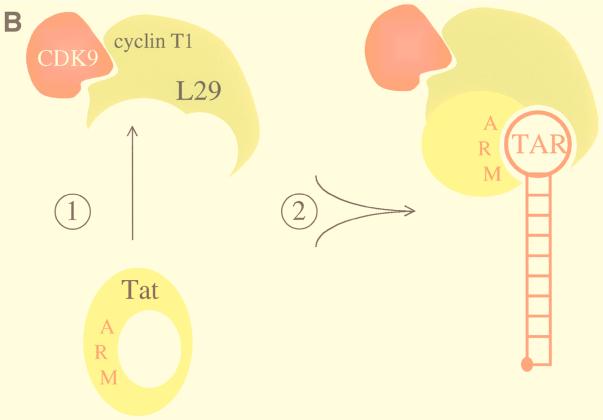
FIG. 7.
Model for the interaction between eTat and eT and the formation of the tripartite complex. (A) The interaction between eTat and eT requires the leucine at position 29 in eT. We propose that eTat does not bind to eTAR because its ARM is occluded by intramolecular interactions involving leucines at positions 50, 54, 74, and 75. Mutations in these residues abolish eTat transactivation (11). Upon binding to eT, intermolecular interactions between these leucines in eTat and leucines at positions 29 (arrow), 35, 44, and 45 in eT open the ARM so that eTat can now interact with eTAR. Shown are N-terminal sequences of eTat and eT in the opposite orientation (arrows from eTat and eT). Also marked are the cysteine-rich and core sequences in eTat. The ARM is contained within gray boxes. Also shown are putative N-terminal and α1 helices of eT (striped bars), which are separated by a loop. Black circles mark proposed leucine bridges. (B) Proposed model for the binding of eTat and eT to eTAR. Upon binding of eTat (yellow) to eT (green, step 1), a combinational surface is formed, consisting of the ARM in eTat and basic residues upstream of the cysteine at position 261 in eT. This surface then binds to eTAR (red line) and recruits CDK9 (red) to the EIAV LTR (step 2). The leucine at position 29 in eT is essential for the intermolecular interaction between eTat and eT. The occluded ARM is represented by a closed circle in eTat. The stem and central loop in eTAR contain 10 paired and 4 unpaired nucleotides, respectively.